Abstract
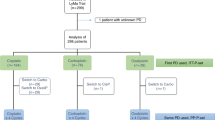
To assess high-dose carboplatin chemotherapy with or without paclitaxel with filgrastim mobilized peripheral blood progenitor cell (PBPC) support in a phase I/II study, a total of 21 patients with mostly chemonaive disease received four cycles of high-dose chemotherapy. Cycle 1 (cyclophosphamide, 6 g/m2) was followed by two cycles of carboplatin (1600 mg/m2 or 1800 mg/m2). Cycle 4 consisted of carboplatin (1600 mg/m2), etoposide (1600 mg/m2), and melphalan (140 mg/m2). Further chemotherapy intensification was achieved by adding paclitaxel (175 mg/m2) to all cycles with a fixed carboplatin dose (1600 mg/m2). Ototoxicity was dose-limiting for escalation of sequential cycles of carboplatin. Grade 2 and grade 3 ototoxicity, hearing loss not requiring a hearing aid, or hearing loss correctable with a hearing aid, was observed with carboplatin at 1800 mg/m2. The maximum tolerated dose (MTD) of sequential carboplatin, therefore, was identified in this study as 1600 mg/m2. After cycles 1, 2, 3 and 4 the median duration of leukopenia (<1.0 × 109/l) was 7, 4, 4 and 6 days. Severe grade 3 and 4 infections were seen in only 7% of cycles. Of the 21 patients evaluable for disease response, 57% had complete remissions and 43% experienced partial remissions resulting in an overall response rate of 100%. The median progression-free survival is 25 (15–36) months, the median overall survial 36.5 (15–38) months. Most patients were suboptimally debulked or had bulky residual disease at the start of chemotherapy. Sequential high-dose chemotherapy to a maximum dose of 1600 mg/m2 carboplatin is effective and feasible. A randomized, prospective trial comparing sequential high-dose chemotherapy with optimal standard chemotherapy is now warranted.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wandt, H., Birkmann, J., Denzel, T. et al. Sequential cycles of high-dose chemotherapy with dose escalation of carboplatin with or without paclitaxel supported by G-CSF mobilized peripheral blood progenitor cells: a phase I/II study in advanced ovarian cancer. Bone Marrow Transplant 23, 763–770 (1999). https://doi.org/10.1038/sj.bmt.1701659
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1701659
Keywords
This article is cited by
-
Printing metal-spiked inks for LA-ICP-MS bioimaging internal standardization: comparison of the different nephrotoxic behavior of cisplatin, carboplatin, and oxaliplatin
Analytical and Bioanalytical Chemistry (2016)
-
Phase II clinical trial with gemcitabine and paclitaxel sequential monotherapy as first-line treatment for advanced non-small-cell lung cancer (SLCG 01-04)
Clinical and Translational Oncology (2011)
-
Hochdosischemotherapie beim fortgeschrittenen Ovarialkarzinom
Der Onkologe (2007)
-
Post-operative sequential high-dose chemotherapy with haematopoietic stem cell support as front-line treatment in advanced ovarian cancer: a phase II multicentre study
Bone Marrow Transplantation (2006)
-
Phase I/II trial of multicycle high-dose chemotherapy with peripheral blood stem cell support for treatment of advanced ovarian cancer
Bone Marrow Transplantation (2006)