Abstract
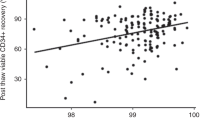
Some of the factors that may influence the number and quality of cord blood haematopoietic progenitor cells available for transplantation have been investigated including site of collection, delayed processing after collection and cryopreservation protocol. We used the granulocyte–macrophage progenitor (CFU-GM) and erythroid burst-forming unit (BFU-E) assays to quantify progenitors. The capacity of CFU-GM to produce secondary colonies was used as a measure of progenitor cell quality. We found that: (1) there were no significant differences in total nucleated cells (TNC), mononuclear cells (MNC), CFU-GM or BFU-E numbers in paired specimens from the umbilical vein or veins at the base of the placenta. The potential of the CFU-GM to produce secondary colonies from the two sites was similar; (2) storing cord blood at room temperature or at 4°C resulted in a significant reduction in progenitor cell numbers beyond 9 h; and (3) cryopreservation following either controlled rate freezing or passive cooling reduced MNC numbers, viability and CFU-GM survival insignificantly but the potential of CFU-GM to produce secondary colonies was significantly reduced post cryopreservation (P = 0.04). We conclude that the yield of CB progenitor cells is not affected by the site of collection, but is adversely affected by delays between collection and cryopreservation. Furthermore, cryo- preservation reduced the CFU-GM potential to produce secondary colonies. Measures of progenitor cell quality as well as quantity may be relevant to assessing CB blood collections.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shlebak, A., Marley, S., Roberts, I. et al. Optimal timing for processing and cryopreservation of umbilical cord haematopoietic stem cells for clinical transplantation. Bone Marrow Transplant 23, 131–136 (1999). https://doi.org/10.1038/sj.bmt.1701551
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1701551
Keywords
This article is cited by
-
Optimum storage conditions for cord blood-derived hematopoietic progenitor cells prior to isolation
Bone Marrow Transplantation (2007)
-
In vitro study on cryopreservation of peripheral blood stem cells with uncontrolled freezer
Chinese Journal of Cancer Research (2002)