Abstract
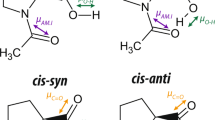
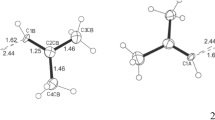
THE accurate crystal structure analyses of α-oxalic acid1 and oxalic acid dihydrate2 show that the length of the central C—C bond is indistinguishable from the single bond-length of 1.5445 A. in diamond within a probable limit of ± 0.025 A. It is inferred that there is no appreciable degree of π-conjugation across the central bond, presumably because of polarization of the carbon 2p electrons by the oxygen atoms; some other explanation must therefore be sought for the fact that the oxalic acid molecule is perfectly flat in both α and β anhydrous forms and in the dihydrate. It seems unlikely that in all three crystal structures the intermolecular hydrogen-bonding system should be specifically that of lowest energy only when the molecules are planar. In both the α anhydrous form and the dihydrate, the C—O bonds are distinguishable in length, one tending towards a double bond, the other towards a single bond. The centre of symmetry of the molecule requires a trans relationship between the bonds as in (I). 
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Cox, E. G., Dougill, Marion and Jeffrey, G. A. (unpublished work).
Ahmed, F., and Cruickshank, D. W. J. (unpublished work).
Stern, F., and Beevers, C. A., Acta Cryst., 3, 341 (1950).
Parry, G. S., Acta Cryst., 4, 131 (1951).
Beevers, C. A., and Hughes, W., Proc. Roy. Soc., A, 177, 251 (1941).
Cochran, W., Acta Cryst., 4, 376 (1951).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
JEFFREY, G., PARRY, G. Evidence for Intramolecular Attraction between Hydroxyl and Carbonyl Oxygen Atoms. Nature 169, 1105–1106 (1952). https://doi.org/10.1038/1691105b0
Issue Date:
DOI: https://doi.org/10.1038/1691105b0
This article is cited by
-
Preparation and properties of peroxy titanium malonate
Proceedings of the Indian Academy of Sciences - Section A (1963)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.