Abstract
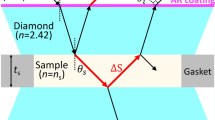
IT was observed by Poiseuille1, Hubener2, Sprüng3, Slotte4 and others, that when substances like ammonium iodide, potassium chloride, etc., are dissolved in water, the viscosity of water is lowered. This phenomenon has since been known as ‘negative viscosity’. The compressibility of aqueous solutions of electrolytes has been studied by Gibson5, Falkenhagen and Bachem6, Szalay7 and others, and they have derived expressions for the relationship between the concentration of electrolyte solutions and their compressibilities. The existing data show that the compressibility continuously decreases as the concentration of electrolytes is increased. But these observations are confined to the positive region of viscosity. In the present communication we record our observations on the ultrasonic velocity in potassium chloride at low concentrations at 24°. The ultrasonic energy was obtained from a quartz disk and was converted into electrical energy by a similar quartz crystal for detection. The apparatus has been described elsewhere8. The slab can be rotated, resulting in a variation in the amplitude of the transmitted energy as recorded on the oscillograph screen. The minimum in the energy is due to the total reflexion of the transmitted energy, as the angle of refraction in the solid is greater than that of incidence. The refractive index at the discontinuity is:  where θl and θs are the angles that the wave-train makes with the normal in the solid and the liquid respectively. Vs is the velocity in the solid, which can be determined in advance by using a liquid with known velocity. At total reflexion of the waves, due to the rotation of the slab,
where θl and θs are the angles that the wave-train makes with the normal in the solid and the liquid respectively. Vs is the velocity in the solid, which can be determined in advance by using a liquid with known velocity. At total reflexion of the waves, due to the rotation of the slab,  A knowledge of θ enables us to determine the velocity in the liquid.
A knowledge of θ enables us to determine the velocity in the liquid.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Poiseuille, Ann. Chem. Phys., 21, 76 (1847).
Hubener, Pogg. Ann., 150, 248 (1878).
Sprüng, A., Pogg. Ann., 159, 1 (1876).
Slotte, K., Weid. Ann., 20, 257 (1883).
Gibson, J. Amer. Chem. Soc., 57, 284 (1935).
Falkenhagen, H., and Bachem, Chem. Nat., 135, 830 (1935); Z. Elektrochem., 41, 570 (1935).
Szalay, A., Phys. Z., 35, 639 (1934).
Srivastava, A. M., Proc. Nat. Acad. Ind., 18, 51 (1949).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
PRAKASH, S., SAXENA, P. & SRIVASTAVA, A. Ultrasonic Velocity in Potassium Chloride Solutions in the Region of their Negative Viscosities. Nature 168, 522–523 (1951). https://doi.org/10.1038/168522a0
Issue Date:
DOI: https://doi.org/10.1038/168522a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.