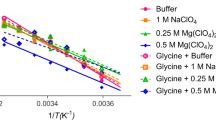
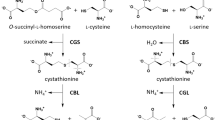
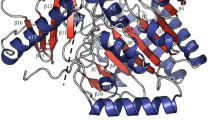
Abstract
THE optimal conditions and specificity of the enzyme sulphatase of ‘clarase’ have been described in a previous communication1. The method then used of measuring the activity of the enzyme by the gravimetric estimation of sulphate liberated from the substrate has now been abandoned, and a new colorimetric method adopted. In this the amount of phenol set free from the substrate (potassium phenylsulphate) in the course of enzymic hydrolysis is determined, as was done by King and Armstrong2 for the determination of phosphatase.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Dzialoszynski, L. M., Nature, 180, 464 (1947).
King, E. T., and Armstrong, A. B., Canad. Med. Assoc. J., 376 (Oct. 1934).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
DZIALOSZYNSKI, L. The Sulphatase of ‘Clarase’: Inhibition, Inactivation and Purification. Nature 166, 157–158 (1950). https://doi.org/10.1038/166157b0
Issue Date:
DOI: https://doi.org/10.1038/166157b0
This article is cited by
-
Examples of Anti-competitive Inhibition
Nature (1956)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.