Abstract
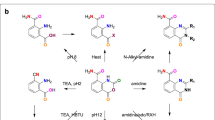
CONDENSATION of nitromeconine and hydrastinine gave a mixture of nitrohydrastines, which was reduced and the product separated into aminohydrastines-a and -b 1. By the removal of the ammo-groups, dl-hydrastine-a (m.p. 137°) and dl-hydrastine-b (m.p. 150–51°) were obtained, but at that time neither of these inactive stereoisomerides could be resolved.
Similar content being viewed by others
Article PDF
References
Hope and Robinson, Proc. Chem. Soc., 28, 17 (1912). Hope, Pyman, Remfry and Robinson, J. Chem. Soc., 236 (1931).
Marshall, Pyman and Robinson, J. Chem. Soc., 1315 (1934).
Cf. Perkin, Rây and Robinson, J. Chem. Soc., 127, 740 (1925).
Groenewoud and Robinson, J. Chem. Soc., 199 (1936).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
HAWORTH, R., PINDER, A. & ROBINSON, R. Synthesis of Hydrastine. Nature 165, 529 (1950). https://doi.org/10.1038/165529a0
Issue Date:
DOI: https://doi.org/10.1038/165529a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.