Abstract
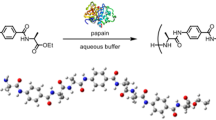
Cook, Heilbron and Levy1 have recently prepared tetraglycylglycine and several di-peptides by reaction of 2-thio-5-thiazolones with amino-acid derivatives. During the past few years, much interest has also been shown in the polymerization potentialities of the N-carboxy anhydrides of α-amino-acids2–5. Study of the reaction of these anhydrides with simple organic bases has now indicated the mechanism of their polymerization and provided a new method of building up polypeptides by addition of single amino-acid units.
Similar content being viewed by others
Article PDF
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
BAILEY, J. A New Peptide Synthesis. Nature 164, 889 (1949). https://doi.org/10.1038/164889a0
Issue Date:
DOI: https://doi.org/10.1038/164889a0
This article is cited by
-
Opening of azole rings by reagents that contain amino groups
Chemistry of Heterocyclic Compounds (1979)
-
Synthesis of poly-L-arginine and the statistical copolymer of L-arginine, L-lysine, and neutral amino acids
Chemistry of Natural Compounds (1966)
-
Kinetics and chemistry of the polycondensation esters of?-amino acids Communication 4. Copolycondensation of glycine ethyl ester and N-carboxyglycine anhydride
Bulletin of the Academy of Sciences of the USSR Division of Chemical Science (1956)
-
Beziehungen zwischen Struktur und chemischer Reaktionsfähigkeit von Wollfasern
Kolloid-Zeitschrift (1951)
-
Anhydro-N-Carboxy-DL-β-Phenylalanine
Nature (1950)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.