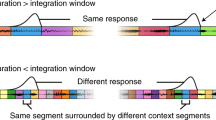
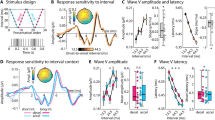
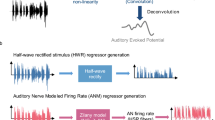
Abstract
Sound-processing strategies that use the highly non-random structure of natural sounds may confer evolutionary advantage to many species. Auditory processing of natural sounds has been studied almost exclusively in the context of species-specific vocalizations1,2,3,4, although these form only a small part of the acoustic biotope5. To study the relationships between properties of natural soundscapes and neuronal processing mechanisms in the auditory system, we analysed sound from a range of different environments. Here we show that for many non-animal sounds and background mixtures of animal sounds, energy in different frequency bands is coherently modulated. Co-modulation of different frequency bands in background noise facilitates the detection of tones in noise by humans, a phenomenon known as co-modulation masking release (CMR)6,7. We show that co-modulation also improves the ability of auditory-cortex neurons to detect tones in noise, and we propose that this property of auditory neurons may underlie behavioural CMR. This correspondence may represent an adaptation of the auditory system for the use of an attribute of natural sounds to facilitate real-world processing tasks.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Pelleg-Toiba, R. & Wollberg, Z. Discrimination of communication calls in the squirrel monkey: “Call detectors” or “cell ensembles”? J. Basic Clin. Physiol. Pharmacol. 2, 257–272 (1991).
Steinschneider, M., Arezzo, J. C. & Vaughan, H. G. J Tonotopic features of speech-evoked activity in primate auditory cortex. Brain Res. 519, 158–168 (1990).
Wang, X., Merzenich, M. M., Beitel, R. & Schreiner, C. E. Representation of a species-specific vocalization in the primary auditory cortex of the common marmoset: temporal and spectral characteristics. J. Neurophysiol. 74, 2685–2706 (1995).
Suga, N. Philosophy and stimulus design for neuroethology of complex-sound processing. Phil. Trans. R. Soc. Lond. B 336, 423–428 (1992).
Aertsen, A. M. H. J., Smolders, J. W. T. & Johannesma, P. I. M. Neural representation of the acoustic biotope: on the existence of stimulus-event relations for sensory neurons. Biol. Cybern. 32, 175–185 (1979).
Hall, J. W., Haggard, M. P. & Fernandes, M. A. Detection in noise by spectro-temporal pattern analysis. J. Acoust. Soc. Am. 76, 50–56 (1984).
Schooneveldt, G. P. & Moore, B. C. Comodulation masking release (CMR) as a function of masker bandwidth, modulator bandwidth, and signal duration. J. Acoust. Soc. Am. 85, 273–281 (1989).
Ruderman, D. L. & Bialek, W. Statistics of natural images: scaling in the woods. Phys. Rev. Lett. 73, 814–817 (1994).
Richards, D. G. & Wiley, R. H. Reverberations and amplitude fluctuations in the propagation of sound in a forest: implication for animal communication. Am. Nat. 115, 381–399 (1980).
Klump, G. M. & Langemann, U. Comodulation masking release in a songbird. Hearing Res. 87, 157–164 (1995).
Rhode, W. S. & Greenwood, D. in Abstracts of the 18th Association for Research in Otolaryngology Meeting 127 (St Petersburg Beach, Florida, (1995)).
Schreiner, C. E. & Urbas, J. V. Representation of amplitude modulation in the auditory cortex of the cat. II. Comparison between cortical fields. Hearing Res. 32, 49–63 (1988).
Rauschecker, J. P., Tian, B. & Hauser, M. Processing of complex sounds in the macaque nonprimary auditory cortex. Science 268, 111–114 (1995).
Eggermont, J. J. Temporal modulation transfer functions for AM and FM stimuli in cat auditory cortex. Effects of carrier type, modulating waveform and intensity. Hearing Res. 74, 51–66 (1994).
Carlyon, R. P., Buus, S. & Florentine, M. Comodulation masking release for three types of modulator as a function of modulation rate. Hearing Res. 42, 37–45 (1989).
Acknowledgements
This work was supported by a grant administered by the Israel Science Foundation. We thank E. Vaadia, M. Abeles, E. Young and A. Aertsen for critical comments to this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nelken, I., Rotman, Y. & Yosef, O. Responses of auditory-cortex neurons to structural features of natural sounds. Nature 397, 154–157 (1999). https://doi.org/10.1038/16456
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/16456
This article is cited by
-
Behind the mask(ing): how frogs cope with noise
Journal of Comparative Physiology A (2023)
-
The role of temporal coherence and temporal predictability in the build-up of auditory grouping
Scientific Reports (2022)
-
Mechanisms of auditory masking in marine mammals
Animal Cognition (2022)
-
Functional Studies of the Primary Auditory Cortex in the Cat
Neuroscience and Behavioral Physiology (2021)
-
Neural encoding and production of functional morphemes in the posterior temporal lobe
Nature Communications (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.