Abstract
Objective:
To evaluate the bifidogenic efficacy of two inulin doses in healthy human adults.
Design:
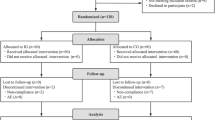
A double-blind, placebo-controlled, crossover human study.
Setting:
Food Microbial Sciences Unit, The University of Reading, Reading, UK.
Subjects:
Thirty healthy volunteers, 15 men, 15 women (age range 19–35).
Interventions:
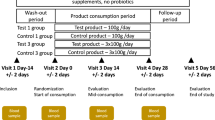
Subjects consumed a chocolate drink containing placebo (maltodextrin, 8 g/day), 5 g/day inulin and 8 g/day inulin for a 2-week treatment period. Each treatment was followed by a 1-week washout at the end of which volunteers progressed to the next treatment. Faecal samples were obtained at the start of the study (baseline) and at the end of each treatment and washout period. Fluorescent in situ hybridization was used to monitor populations of Bifidobacterium genus, Bacteroides – Prevotella, Lactobacillus – Enterococcus and Clostridium perfringens – histolyticum subgroup.
Results:
Bifidobacterial levels increased significantly upon ingestion of both the low (9.78±0.29 log10 cells/g faeces, P<0.05) and the high inulin dose (9.79±0.38 log10 cells/g faeces, P=0.05) compared to placebo (9.64±0.23 log10 cells/g faeces).
Conclusions:
Both inulin doses exhibited a bifidogenic effect but a higher volunteer percentage responded to the high dose. A dose response effect was not observed but the magnitude of increase in bifidobacteria levels depended on their initial numbers. The higher the initial concentrations the smaller was the increase upon ingestion of the active treatments.
Sponsorship:
Financial support for the completion of this project was provided by Sensus (Roosendaal, The Netherlands).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Bernet MF, Brassart D, Neeser JR, Servin AL (1993). Adhesion of human bifidobacterial strains to cultured human intestinal epithelial cells and inhibition of enteropathogen-cell interactions. Appl Environ Microbiol 59, 4121–4128.
Bouhnik Y, Raskine L, Simoneau G, Vicaut E, Neut C, Flourie B et al. (2004). The capacity of nondigestible carbohydrates to stimulate fecal bifidobacteria in healthy humans: a double-blind, randomized. Placebo-controlled, parallel-group, dose response relation study. Am J Clin Nutr 80, 1658–1664.
Bouhnik Y, Vahedi K, Achour L, Attar A, Salfati J, Pochart P et al. (1999). Short-chain fructo-oligosaccharide administration dose dependently increases fecal bifidobacteria in healthy humans. J Nutr 129, 113–116.
Buddington RK, Williams CH, Chen SC, Witherly SA (1996). Dietary supplement of neosugar alters the faecal flora and decreases the activities of some reductive enzymes in human subjects. Am J Clin Nutr 63, 709–716.
Campbell H, Jones I (1996). Promoting breastfeeding: a view of the current position and a proposed agenda for action in Scotland. J Public Health Med 18, 406–414.
Collins MD, Gibson GR (1999). Probiotics, prebiotics and synbiotics: approaches for the nutritional modulation of microbial ecology. Am J Clin Nutr 69, 1052s–1057s.
Den Hond E, Geypens B, Ghoos Y (2000). Effect of high performance chicory inulin on constipation. Nutr Res 20, 731–736.
Engfer MB, Stahl B, Finke B, Sawatzki G, Daniel H (2000). Human milk oligosaccharides are resistant to enzymatic hydrolysis in the upper gastrointestinal tract. Am J Clin Nutr 71, 1589–1596.
Franks AH, Harmsen HJM, Raangs GC, Jansen GJ, Schut F, Welling GW (1998). Variations of bacterial populations in human faeces measured by fluorescent in situ hybridisation with group-specific 16S rRNA-targeted oligonucleotide probes. Appl Environ Microb 64, 3336–3345.
Gibson GR, Beatty ER, Wang X, Cummings JH (1995). Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology 108, 975–982.
Gibson GR, Wang X (1994). Enrichment of bifidobacteria from human gut contents by oligofructose using continuous culture. FEMS Microbiol Lett 118, 121–128.
Harmsen HJM, Elfferich P, Schut F, Welling GW (1999). A 16S rRNA-targeted probe for detection of lactobacilli and enterococci in faecal samples by fluorescent in situ hybridization. Microb Ecol Health D 11, 3–12.
Kleessen B, Sykura B, Zunft HJ, Blaut M (1997). Effects of inulin and lactose on faecal microflora, microbial activity and bowel habit in elderly constipated persons. Am J Clin Nutr 65, 1397–1402.
Kruse HP, Kleessen B, Blaut M (1999). Effects of inulin on faecal bifidobacteria in human subjects. Brit J Nutr 82, 375–382.
Langendijk PS, Schut F, Jansen GJ, Raangs GW, Kamphuis GR, Wilkinson MHF et al. (1995). Quantitative fluorescent in situ hybridization of Bifidobacterium spp. with genus-specific 16S rRNA-targeted probes and its application in faecal samples. Appl Environ Microb 61, 3069–3075.
Manz W, Amann R, Ludwig W, Vancanneyt M, Schleifer K-H (1996). Application of a suite of 16S rRNA-specific oligonucleotide probes designed to investigate bacteria of the phylum cytophaga-flavobacter-bacteroides in the natural environment. Microbiology 142, 1097–1106.
McCracken VJ, Gaskins HR (1999). Probiotics and the immune system. In: Tannock GW (ed). Probiotics: A Critical Review. Horizon Scientific Press: Wymondham. pp 85–111.
Moshfegh AJ, Friday JE, Goldman JP, Chug Ahuja JK (1999). Presence of inulin and oligofructose in the diets of Americans. J Nutr 129 (Suppl), S1407–S1411.
Roberfroid MB, Van Loo JAE, Gibson GR (1998). The bifidogenic nature of chicory inulin and its hydrolysis products. J Nutr 128, 1–9.
Rycroft CE, Jones MR, Gibson GR, Rastall RA (2001). A comparative in vitro evaluation of the fermentation properties of prebiotic oligosaccharides. J Appl Microb 91, 878–887.
Saavedra JM, Bauman NA, Oung I, Perman JA, Yolken RH (1994). Feeding of Bifidobacterium bifidum and Streptococcus thermophilus to infants in hospital for prevention of diarrhoea and shedding of rotavirus. Lancet 344, 1046–1049.
Tuohy KM, Finlay RK, Wynne AG, Gibson GR (2001a). A human volunteer study on the probiotic effects of HP-inulin-faecal bacteria enumerated using fluorescent in situ hybridisation (FISH). Anaerobe 7, 113–118.
Tuohy KM, Kolida S, Lustenberger A, Gibson GR (2001b). The prebiotic effects of biscuits containing partially hydrolyzed guar gum and fructooligosaccharides – a human volunteer study. Brit J Nutr 86, 341–348.
Van Loo J, Coussement P, De Leenheer L, Hoebregs H, Smits G (1995). On the presence of inulin and oligofructose as natural ingredients in the Western diet. Crit Rev Food Sci Nutr 35, 525–552.
Vanderhoof JA, Young RJ (1998). Use of probiotics in childhood gastrointestinal disorders. J Pediatr Gastr Nutr 27, 323–332.
Williams CH, Witherly SA, Buddington RK (1994). Influence of dietary Neosugar on selected bacteria groups of the human fecal microbiota. Microb Ecol Health Dis 7, 91–97.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kolida, S., Meyer, D. & Gibson, G. A double-blind placebo-controlled study to establish the bifidogenic dose of inulin in healthy humans. Eur J Clin Nutr 61, 1189–1195 (2007). https://doi.org/10.1038/sj.ejcn.1602636
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ejcn.1602636
Keywords
This article is cited by
-
The hallmarks of dietary intervention-resilient gut microbiome
npj Biofilms and Microbiomes (2022)
-
Validity of food additive maltodextrin as placebo and effects on human gut physiology: systematic review of placebo-controlled clinical trials
European Journal of Nutrition (2022)
-
Dietary calcium phosphate strongly impacts gut microbiome changes elicited by inulin and galacto-oligosaccharides consumption
Microbiome (2021)
-
Dietary fibre in gastrointestinal health and disease
Nature Reviews Gastroenterology & Hepatology (2021)
-
Novel fructooligosaccharides of Dioscorea alata L. tuber have prebiotic potentialities
European Food Research and Technology (2021)