Abstract
Objective:
To assess the effect of a 4-week herring diet compared to a reference diet on biomarkers for cardiovascular disease in obese subjects.
Design:
Randomized crossover trial.
Setting:
Department of Internal Medicine, Sahlgrenska University Hospital.
Subjects:
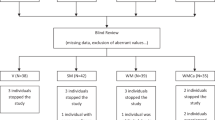
Fifteen healthy obese men and women (age 24–70 years) included, 13 completed.
Intervention:
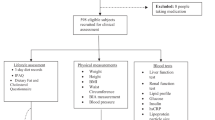
Subjects were randomly assigned to four weeks of herring diet (150 g baked herring fillets/day 5, days/week) or reference diet (pork and chicken fillets) and switched diets after 2 weeks washout. P-total cholesterol, p-TAG, p-HDL, p-HDL2, p-HDL3, p-LDL, p-apolipoprotein A, p-apolipoprotein B, p-Lipoprotein (a), p-fibrinogen, p-C- reactive protein and p-antioxidative capacity were analysed at 0,2,4,6,8 and 10 weeks.
Results:
P-HDL was significantly higher after the herring diet period compared to after the reference diet period; 1.22 vs 1.13 mmol/l (P=0.036). There was a small, but not statistically significant, decrease in TAG but no effect on other biomarkers. TEAC and FRAP, but not ORAC-values, indicated that plasma antioxidants may have been reduced. CRP tended to be lower after the herring diet compared to after the reference diet.
Conclusions:
Consumption of oven-baked herring (150g/day, 5 days/week) for 4 weeks, compared to consumption of pork and chicken fillets, significantly increased p-HDL. Patients with insulin resistance and obesity, who commonly have low HDL, may therefore benefit from addition of herring to the diet.
Sponsorship:
Region Västra Götaland, National board of fisheries (Dr 223-2451-01), Sweden (EU structural funds), The Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (FORMAS) (Grant No 2001-1246).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Anttolainen M, Valsta LM, Alfthan G, Kleemola P, Salminen I, Tamminen M (1996). Effect of extreme fish consumption on dietary and plasma antioxidant levels and fatty acid composition. Eur J Clin Nutr 50, 741–746.
Benzie IF, Strain JJ (1996). The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’: the FRAP assay. Anal Biochem 239, 70–76.
Bezard J, Blond JP, Bernard A, Clouet P (1994). The metabolism and availability of essential fatty acids in animal and human tissues. Reprod Nutr Dev 34, 539–568.
Bligh EG, Dyer WJ (1959). A rapid method of total lipid extraction and purification. Can J Biochem Phys 37, 911–917.
Brown AJ, Roberts DC, Pritchard JE, Truswell AS (1990). A mixed Australian fish diet and fish-oil supplementation: impact on the plasma lipid profile of healthy men. Am J Clin Nutr 52, 825–833.
Brown AJ, Roberts DCK (1991). Fish and fish oil intake: effect on haematological variables related to cardiovascular disease. Thromb Res 64, 169–178.
Chan DC, Watts GF (2003). Randomized controlled trial or the effect of n-3 fatty acid supplementation on the metabolism of apolipoprotein B-100 and chylomicron remnants in men with visceral obesity. Am J Clin Nutr 77, 300–307.
Clauss A (1957). Rapid physiological coagulation method for the determination of fibrinogen. Acta Haematol 17, 237–246.
Cobiac L, Clifton PM, Abbey M, Belling GB, Nestel PJ (1991). Lipid, lipoprotein, and hemostatic effects of fish vs fish-oil n-3 fatty acids in mildly hyperlipidemic males. Am J Clin Nutr 53, 1210–1216.
Davalos A, Gomez-Cordoves C, Bartolome B (2004). Extending applicability of the oxygen radical absorbance capacity (ORAC-fluorescein) assay. J Agric Food Chem 52, 48–54.
Elvevoll EO, Österud B (2003). Impact of processing on nutritional quality of marine food items. In Elmadfa I AE, Konig JS (eds). Modern aspects of marine food items-present knowledge and future perspectives. Karger Press: Basel. pp 337–341.
Fehily AM, Burr ML, Phillips KM, Deadman NM (1983). The effect of fatty fish on plasma lipid and lipoprotein concentrations. Am J Clin Nutr 38, 349–351.
Fiskeriverket (2001). Betydelsen av de kvoter som står till det svenska yrkesfiskets förfogande. Report to the Swedish Government, Dnr 121-2351-00. National Board of Fisheries: Göteborg.
Fiskeriverket (2002). Fakta om svenskt fiske och fiskkonsumtion, Göteborgs Lånstryckeri AB, Göteborg 2003.
Friedewald WT, Lecy RI (1972). Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18, 499–502.
Gerhard GT, Patton BD, Lindquist SA, Wander RC (1991). Comparison of three species of dietary fish: effects on serum concentrations of low-density-lipoprotein cholesterol and apolipoprotein in normotriglyceridemic subjects. Am J Clin Nutr 54, 334–339.
Gidez LI, Miller GJ (1982). Separation and quantitation of subclasses of human plasma high density lipoproteins by a simple precipitation procedure. J Lipid Res 23, 1206–1223.
Gordoa JC, Renobales M (2002). Habitual fish intake is associated with decreased LDL susceptibility to ex vivo oxidation. Lipids 37, 333–341.
Gordon T, Castelli WP (1977). High density lipoprotein as a protective factor against coronary heart disease- the Framingham study. Am JMed 62, 707–714.
Hagenfeldt L (1975). Turnover of individual free fatty acids in man. Fed Proc 34, 2246–2249.
Hallgren CG, Hallmans G, Jansson J-H, Marklund SL, Huhtasaari F, Schutz A et al. (2001). Markers for high fish intake are associated with decreased risk of first myocardial infarction. Br J Nutr 86, 397–404.
Harris WS (1997). n-3 fatty acids and serum lipoproteins: human studies. Am J Clin Nutr 65 (Suppl), 1645S–1654S.
Hänninen OO, Ågren JJ (1989). Effects of moderate fresh water fish diet on lipid metabolism of Finnish students. J Int Med 225, 77–81.
Jacques H, Gascon A, Bergeron N, Lavigne C, Hurley C, Deshaines Y et al. (1995). Role of dietary fish protein in the regulation of plasma lipids. Can J Cardiol 11S, 63G–71G.
Jacques H, Noreau L, Moorjani S (1992). Effects on plasma lipoprteins and exogenous sex hormones of substituting lean white fish for other animal-protein sources in diets of postmenopausal women. Am J Clin Nutr 55, 896–901.
Jansen S, Lopez-Miranda J (1998). Plasma lipid response to hypolipidemic diets in young healthy non-obese men varies with body mass index. J Nut 128, 1144–1149.
Kris-Etherton PM, Harris WS, Appel LJ, Association ANCAH (2002). Fish consumption, fish oil, omega-3 fatty acids and cardiovascular disease. Circulation 106, 2747–2757.
Kromhout D, Bosschieter EB, de Lezenne Coulander C (1985). The inverese relation between fish consumption and 20-year mortality from coronary heart disease. New Engl J Med 312, 1205–1209.
Lee C, Trevino B, Chaiyawat M (1996). A simple and rapid solvent extraction method for determining total lipids in fish tissue. J AOAC Int 79, 487–492.
Lepage G, Roy CC (1986). Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res 27, 114–120.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951). Protein measurement with the folin phenol reagent. J Biol Chem 193, 265–275.
Lundberg S (2004). Fisk till middag: WWF:s konsumentguide för miljövänligare köp av fisk- och skaldjurs produkter, 3rd Edn, World Wide Fund for nature.
Markwell MK, Haas SM, Bieber LL, Tolbert NE (1978). Modification of Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem 87, 206–210.
Miller GJ, Miller NJ (1975). Plasma high-density lipoprotein concentration and the development of ischemic heart disease. Lancet i, 16–19.
Mori TA, Vandongen R, Beilin LJ, Burke V, Morris J, Ritchie J (1994). Effects of varying dietary fat, fish, and fish oils on blood lipids in a randomized controlled trial in men at risk of heart disease. Am J Clin Nutr 59, 1060–1068.
Mozaffarian D (2005). Fish intake and risk of incident heart failure. J Am Coll Cardiol 45, 2015–2021.
Pawlosky R, Hibbeln J, Lin Y, Salem NJ (2003). n-3 fatty acid metabolism in women. Br J Nutr 90, 993–995.
Regulska-Ilow B, Ilow R (2002). Comparison of the effects of microwave cooking and conventional cooking methods on the composition of fatty acids and fat quality indicators in herring. Nahrung/Food 46, 383–388.
Savige GS (2001). Candidate foods in the Asia-pacific region for cardiovascular protection: fish, fruit and vegetables. Asia Pac J Clin Nutr 10, 134–137.
Simopoulos AP (1997). Nutritional aspects of fish. In Luten JB, Borrensen T, Oehlenschläger J (eds). Seafood from producer to consumer. Elsevier Science: Amsterdam. pp 589–607.
Tidwell DK, McNaughton JP, Pellum LK, McLaurin BP, Chen S-C (1993). Comparison of the effects of adding fish high or low in n-3 fatty acids to a diet conforming to the dietary guidelines for Americans. J Am Diet Ass 93, 1124–1128.
Tuominen T-R, Esmark M (2003). Food for thought: the use of marine resources in fish feed. Institue of Marine Research: Tromso.
Undeland I, Ellegard L, Sandberg A-S (2004). Fish and cardiovascular health. Scand J Nut 48, 119–130.
van Houwelingen AC, Nordöy A, van der Beek E, Houtsmuller U, de Metz M, Hornstra G (1987). Effect of a moderate fish intake on blood pressure, bleeding time, hematology, and clinical chemistry in healthy males. Am J Clin Nutr 46, 424–436.
Wolmarans P, Labadarios D, Benade AJ, Kotze TJ, Luow ME (1993). The influence of consuming fatty fish instead of red meat on plasma levels of vitamins A, C and E. Eur J Clin Nutr 47, 97–103.
Acknowledgements
The herring fillets were supported by Paul Mattsson AB, Ellös, Sweden. Thanks to Annette Almgren for help with both the food preparation and blood sampling and to Nils-Gunnar Carlsson for the methods for fatty acid analysis. Special thanks to Angela Silveira at Karolinska Hospital (Stockholm), who made it possible for us to analyze fibrinogen in the plasma samples.
Author information
Authors and Affiliations
Corresponding author
Additional information
Contributors and Guarantors: H Lindqvist, AS Sandberg, AM Langkilde and I Undeland.
Rights and permissions
About this article
Cite this article
Lindqvist, H., Langkilde, A., Undeland, I. et al. Herring (Clupea harengus) supplemented diet influences risk factors for CVD in overweight subjects. Eur J Clin Nutr 61, 1106–1113 (2007). https://doi.org/10.1038/sj.ejcn.1602630
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ejcn.1602630
Keywords
This article is cited by
-
Biomarkers of meat and seafood intake: an extensive literature review
Genes & Nutrition (2019)
-
Dietary marine-derived long-chain monounsaturated fatty acids and cardiovascular disease risk: a mini review
Lipids in Health and Disease (2016)
-
Postprandial lipid and insulin responses among healthy, overweight men to mixed meals served with baked herring, pickled herring or baked, minced beef
European Journal of Nutrition (2015)