Abstract
Objective:
To determine current practice in the delivery of parenteral nutrition (PN) in Australian hospitals.
Design:
A cross-sectional mail survey.
Setting:
Acute-care adult hospitals with greater than 200 beds in Australia.
Subjects:
A total of 67 hospitals (65.7% response rate).
Intervention:
Surveys were posted to hospitals. A reminder letter with a second copy of the survey was posted 3 weeks later to non-respondents.
Results:
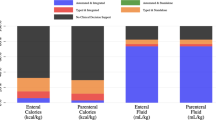
Twenty-seven (40.3%) of the hospitals have a PN team and 50 (74.6%) have a hospital protocol for PN delivery. An inaccessible or non-functional gastrointestinal tract is the most common indicator for commencing PN. Fat infusion is calculated by 24 (38.7%) respondents with a mean (s.d.) maximum amount of fat provided of 2.0 (0.7) g/kg/day. Over half (n=35) reported calculating carbohydrate infusion at a maximum amount of 5.4 (1.0) mg/kg/min. Two-thirds (n=41) reported commencing PN at a rate of 50% or less of goal rate. Blood glucose levels (BGL) were monitored at least once per day by the majority of respondents (n=56, 83.6%). Insulin infusion was commenced at varying BGL. Most respondents (n=40, 59.7%) reported ceasing PN when at least half of the patient's requirements are being met either orally or enterally. A number of practice guidelines were identified and the results of the survey were compared with these guidelines.
Conclusions:
Where there are clear practice guidelines, current practice appears to be in line with these recommendations, however, where evidence is lacking, practice is varied.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
ASPEN Board of Directors and The Clinical Guidelines Task Force (2002). Guidelines for the use of parenteral and enteral nutrition in adult and pediatric patients. JPEN 26, 1S–138S.
American Gastroenterological Association (2001). American Gastroenterological Association medical position statement: parenteral nutrition. Gastroenterology 121, 966–969.
Australasian Society for Parenteral and Enteral Nutrition (1999). AuSPEN Guidelines for Intravenous Trace Elements and Vitamins. AuSPEN: Melbourne.
British Association for Parenteral and Enteral Nutrition Working Party (1996). Current Perspectives on Parenteral Nutrition in Adults. BAPEN: Berks.
British Society of Gastroenterology (1996). Guidelines for Artificial Nutrition Support. British Society of Gastroenterology: London.
Buchman A (2002). Total parenteral nutrition-associated liver disease. JPEN 26, S43–S48.
Cavicchi M, Beau P, Crenn P, Degott C, Messing B (2000). Prevalence of liver disease and contributing factors in patients receiving home parenteral nutrition for permanent intestinal failure. Ann Intern Med 132, 525–532.
Chan S, McCowen KC, Bistrian BR, Thibault A, Keane-Ellison M, Forse RA et al. (1999). Incidence, prognosis, and etiology of end-stage liver disease in patients receiving home total parenteral nutrition. Surgery 126, 28–34.
Crook MA, Hally V, Panteli JV (2001). The importance of the refeeding syndrome. Nutrition 17, 632–637.
Druml W, Fischer M, Ratheiser K (1998). Use of intravenous lipids in critically ill patients with sepsis without and with hepatic failure. JPEN 22, 217–223.
Dudrick SJ, Macfadyen Jr BV, Van Buren CT, Ruberg RL, Maynard AT (1972). Parenteral hyperalimentation. Metabolic problems and solutions. Ann Surg 176, 259–264.
Duggan C, Rizzo C, Cooper A, Klavon S, Fuchs V, Gura K et al. (2002). Effectiveness of a clinical practice guideline for parenteral nutrition: a 5-year follow-up study in a pediatric teaching hospital. JPEN 26, 377–381.
Fischer GW, Hunter KW, Wilson SR, Mease AD (1980). Diminished bacterial defences with intralipid. Lancet 2, 819–820.
Fraser I, Neoptolemos J, Woods P (1983). The effect of intralipid on human lymphocyte and monocyte function. Clin Nutr 2, 37–40.
Garcia de Lorenzo A, Lopez-Martinez J, Planas M, Chacon P, Montejo JC, Bonet A et al. (2003). Safety and metabolic tolerance of a concentrated long-chain triglyceride lipid emulsion in critically ill septic and trauma patients. JPEN 27, 208–215.
Guenst JM, Nelson LD (1994). Predictors of total parenteral nutrition-induced lipogenesis. Chest 105, 553–559.
Ioannides-Demos LL, Liolios L, Topliss DJ, McLean AJ (1995). A prospective audit of total parenteral nutrition at a major teaching hospital. Med J Aust 163, 233–237.
Kalfarentzos F, Kokkinis K, Leukaditi K, Maroulis J, Onoufriou A, Alexopoulos K (1998). Comparison between two fat emulsions: intralipid 30% vs intralipid 10% in critically ill patients. Clin Nutr 17, 31–34.
Marhoffer W, Stein M, Maeser E, Federlin K (1992). Impairment of polymorphonuclear leukocyte function and metabolic control of diabetes. Diabetes Care 15, 256–260.
Monson JFT, Ramsden CW, MacFie J, Brennan TG, Guillou PJ (1986). Immunorestorative effect of lipid emulsions during total parenteral nutrition. Br J Surg 73, 843–846.
Palmblad J (1991). Intravenous lipid emulsions and host defense – a critical review. Clin Nutr 10, 303–308.
Reeves MM, Capra S (2003). Variation in the application of methods used for predicting energy requirements in acutely ill adult patients: a survey of practice. Eur J Clin Nutr 57, 1530–1535.
Rosmarin DK, Wardlaw GM, Mirtallo J (1996). Hyperglycemia associated with high, continuous infusion rates of total parenteral nutrition dextrose. Nutr Clin Pract 11, 151–156.
Rossini AA (1976). Why control blood glucose levels? Arch Surg 111, 229–233.
Solomon SM, Kirby DF (1990). The refeeding syndrome: a review. JPEN 14, 90–97.
Tay SM, Ip-Yam PC, Lim BL, Chan YW (2002). Audit of total parenteral nutrition in adult surgical intensive care. Ann Acad Med Singapore 31, 487–492.
The Australian Hospitals Directory (2000): ATA Professional Services Pty Ltd: North Sydney.
The EAST Practice Management Guidelines Workgroup (2003). Practice Management Guidelines for Nutritional Support of the Trauma Patient. Eastern Association for the Surgery of Trauma (EAST): Winston-Salem.
Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M et al. (2001). Intensive insulin therapy in critically ill patients. N Engl J Med 345, 1359–1367.
Wolfe BM, Mathiesen KA (1997). Clinical practice guidelines in nutrition support: can they be based on randomized clinical trials? JPEN 21, 1–6.
Woodcock NP, Zeigler D, Palmer D, Buckley P, Mitchell CJ, MacFie J (2001). Enteral versus parenteral nutrition: a pragmatic study. Nutrition 17, 1–12.
Acknowledgements
We thank all the respondents who willingly completed the surveys. We also thank Dr Peter Kruger and Dr Andrew Pascoe for assistance with proof-reading and editing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guarantor: A Ali.
Contributors: ABA initiated and designed the study, carried out data collection and assisted with the interpretation and discussion of results, and writing of the manuscript. CCK initiated the study and assisted in the design of the study and interpretation and discussion of results. MMR carried out data analysis and was responsible for the writing of the manuscript.
Rights and permissions
About this article
Cite this article
Ali, A., Chapman-Kiddell, C. & Reeves, M. Current practices in the delivery of parenteral nutrition in Australia. Eur J Clin Nutr 61, 554–560 (2007). https://doi.org/10.1038/sj.ejcn.1602547
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ejcn.1602547