Abstract
Objective:
To compare the effects of alpha-linolenic acid (ALA, C18:3n-3) to those of eicosapentaenoic acid (EPA, C20:5n-3) plus docosahexaenoic acid (DHA, C22:6n-3) on cardiovascular risk markers in healthy elderly subjects.
Design:
A randomized double-blind nutritional intervention study.
Setting:
Department of Human Biology, Maastricht University, the Netherlands.
Subjects:
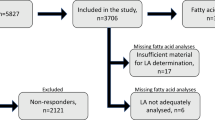
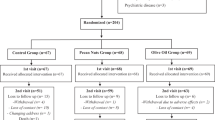
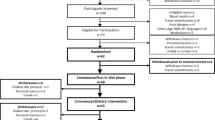
Thirty-seven mildly hypercholesterolemic subjects, 14 men and 23 women aged between 60 and 78 years.
Interventions:
During a run-in period of 3 weeks, subjects consumed an oleic acid-rich diet. The following 6 weeks, 10 subjects remained on the control diet, 13 subjects consumed an ALA-rich diet (6.8 g/day) and 14 subjects an EPA/DHA-rich diet (1.05 g EPA/day+0.55 g DHA/day).
Results:
Both n-3 fatty acid diets did not change concentrations of total-cholesterol, LDL-cholesterol, HDL-cholesterol, triacylglycerol and apoA-1 when compared with the oleic acid-rich diet. However, after the EPA/DHA-rich diet, LDL-cholesterol increased by 0.39 mmol/l (P=0.0323, 95% CI (0.030, 0.780 mmol/l)) when compared with the ALA-rich diet. Intake of EPA/DHA also increased apoB concentrations by 14 mg/dl (P=0.0031, 95% CI (4, 23 mg/dl)) and 12 mg/dl (P=0.005, 95% CI (3, 21 mg/dl)) versus the oleic acid and ALA-rich diet, respectively. Except for an EPA/DHA-induced increase in tissue factor pathway inhibitor (TFPI) of 14.6% (P=0.0184 versus ALA diet, 95% CI (1.5, 18.3%)), changes in markers of hemostasis and endothelial integrity did not reach statistical significance following consumption of the two n-3 fatty acid diets.
Conclusions:
In healthy elderly subjects, ALA might affect concentrations of LDL-cholesterol and apoB more favorably than EPA/DHA, whereas EPA/DHA seems to affect TFPI more beneficially.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Allman-Farinelli MA, Hall D, Kingham K, Pang D, Petocz P, Favaloro EJ (1999). Comparison of the effects of two low fat diets with different alpha-linolenic:linoleic acid ratios on coagulation and fibrinolysis. Atherosclerosis 142, 159–168.
Assmann G, Carmena R, Cullen P, Fruchart JC, Jossa F, Lewis B et al. (1999). Coronary heart disease: reducing the risk: a worldwide view. International Task Force for the Prevention of Coronary Heart Disease. Circulation 100, 1930–1938.
Baro L, Fonolla J, Pena JL, Martinez-Ferez A, Lucena A, Jimenez J et al. (2003). n-3 Fatty acids plus oleic acid and vitamin supplemented milk consumption reduces total and LDL cholesterol, homocysteine and levels of endothelial adhesion molecules in healthy humans. Clin Nutr 22, 175–182.
Berrettini M, Parise P, Ricotta S, Iorio A, Peirone C, Nenci GG (1996). Increased plasma levels of tissue factor pathway inhibitor (TFPI) after n-3 polyunsaturated fatty acids supplementation in patients with chronic atherosclerotic disease. Thromb Haemost 75, 395–400.
Berstad P, Seljeflot I, Veierod MB, Hjerkinn EM, Arnesen H, Pedersen JI (2003). Supplementation with fish oil affects the association between very long-chain n-3 polyunsaturated fatty acids in serum non-esterified fatty acids and soluble vascular cell adhesion molecule-1. Clin Sci (London) 105, 13–20.
Burdge G (2004). Alpha-linolenic acid metabolism in men and women: nutritional and biological implications. Curr Opin Clin Nutr Metab Care 7, 137–144.
Clauss A (1957). Rapid physiological coagulation method in determination of fibrinogen. Acta Haematol 17, 237–246.
de Lorgeril M, Renaud S, Mamelle N, Salen P, Martin JL, Monjaud I et al. (1994). Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet 343, 1454–1459.
Emken EA, Adlof RO, Gulley RM (1994). Dietary linoleic acid influences desaturation and acylation of deuterium-labeled linoleic and linolenic acids in young adult males. Biochim Biophys Acta 1213, 277–288.
Finnegan YE, Howarth D, Minihane AM, Kew S, Miller GJ, Calder PC et al. (2003a). Plant and marine derived (n-3) polyunsaturated fatty acids do not affect blood coagulation and fibrinolytic factors in moderately hyperlipidemic humans. J Nutr 133, 2210–2213.
Finnegan YE, Minihane AM, Leigh-Firbank EC, Kew S, Meijer GW, Muggli R et al. (2003b). Plant- and marine-derived n-3 polyunsaturated fatty acids have differential effects on fasting and postprandial blood lipid concentrations and on the susceptibility of LDL to oxidative modification in moderately hyperlipidemic subjects. Am J Clin Nutr 77, 783–795.
Freese R, Mutanen M (1997). Alpha-linolenic acid and marine long-chain n-3 fatty acids differ only slightly in their effects on hemostatic factors in healthy subjects. Am J Clin Nutr 66, 591–598.
Friedewald WT, Levy RI, Fredrickson DS (1972). Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18, 499–502.
GISSI-Prevenzione-Investigators (1999). Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet 354, 447–455.
Goyens PL, Spilker ME, Zock PL, Katan MB, Mensink RP (2005). Compartmental modeling to quantify alpha-linolenic acid conversion after longer term intake of multiple tracer boluses. J Lipid Res 46, 1474–1483.
Grundt H, Nilsen DW, Hetland O, Mansoor MA, Aarsland T, Woie L (1999). Atherothrombogenic risk modulation by n-3 fatty acids was not associated with changes in homocysteine in subjects with combined hyperlipidaemia. Thromb Haemost 81, 561–565.
Hansen J, Grimsgaard S, Nordoy A, Bonaa KH (2000). Dietary supplementation with highly purified eicosapentaenoic acid and docosahexaenoic acid does not influence PAI-1 activity. Thromb Res 98, 123–132.
Harris WS (1997). n-3 fatty acids and serum lipoproteins: human studies. Am J Clin Nutr 65, 1645S–1654S.
Harris WS (2001). Omega-3 long-chain PUFA and triglyceride lowering: minimum effective intakes. Eur Heart J Suppl 3, D59–D61.
Hjerkinn EM, Seljeflot I, Ellingsen I, Berstad P, Hjermann I, Sandvik L et al. (2005). Influence of long-term intervention with dietary counseling, long-chain n-3 fatty acid supplements, or both on circulating markers of endothelial activation in men with long-standing hyperlipidemia. Am J Clin Nutr 81, 583–589.
Hornstra G (2001). Influence of dietary fat type on arterial thrombosis tendency. J Nutr Health Aging 5, 160–166.
Lemaitre RN, King IB, Mozaffarian D, Kuller LH, Tracy RP, Siscovick DS (2003). n-3 Polyunsaturated fatty acids, fatal ischemic heart disease, and nonfatal myocardial infarction in older adults: the Cardiovascular Health Study. Am J Clin Nutr 77, 319–325.
Miles EA, Thies F, Wallace FA, Powell JR, Hurst TL, Newsholme EA et al. (2001). Influence of age and dietary fish oil on plasma soluble adhesion molecule concentrations. Clin Sci (London) 100, 91–100.
Miller GJ (2005). Dietary fatty acids and the haemostatic system. Atherosclerosis 179, 213–227.
Mozaffarian D, Kumanyika SK, Lemaitre RN, Olson JL, Burke GL, Siscovick DS (2003a). Cereal, fruit, and vegetable fiber intake and the risk of cardiovascular disease in elderly individuals. Jama 289, 1659–1666.
Mozaffarian D, Lemaitre RN, Kuller LH, Burke GL, Tracy RP, Siscovick DS (2003b). Cardiac benefits of fish consumption may depend on the type of fish meal consumed: the Cardiovascular Health Study. Circulation 107, 1372–1377.
Pawlosky RJ, Hibbeln JR, Novotny JA, Salem Jr N (2001). Physiological compartmental analysis of alpha-linolenic acid metabolism in adult humans. J Lipid Res 42, 1257–1265.
Rallidis LS, Paschos G, Papaioannou ML, Liakos GK, Panagiotakos DB, Anastasiadis G et al. (2004). The effect of diet enriched with alpha-linolenic acid on soluble cellular adhesion molecules in dyslipidaemic patients. Atherosclerosis 174, 127–132.
Sanders TA (1996). Effects of unsaturated fatty acids on blood clotting and fibrinolysis. Curr Opin Lipidol 7, 20–23.
Sandset PM, Abildgaard U, Pettersen M (1987). A sensitive assay of extrinsic coagulation pathway inhibitor (EPI) in plasma and plasma fractions. Thromb Res 47, 389–400.
Stichting Nevo (1989). NEVO tabel, Nederlands voedingsstoffenbestand (Dutch food composition table). Voorlichtingsbureau voor de voeding: Den Haag.
Temme EH, Mensink RP, Hornstra G (1999). Effects of diets enriched in lauric, palmitic or oleic acids on blood coagulation and fibrinolysis. Thromb Haemost 81, 259–263.
Thies F, Miles EA, Nebe-von-Caron G, Powell JR, Hurst TL, Newsholme EA et al. (2001). Influence of dietary supplementation with long-chain n-3 or n-6 polyunsaturated fatty acids on blood inflammatory cell populations and functions and on plasma soluble adhesion molecules in healthy adults. Lipids 36, 1183–1193.
Wensing AG, Mensink RP, Hornstra G (1999). Effects of dietary n-3 polyunsaturated fatty acids from plant and marine origin on platelet aggregation in healthy elderly subjects. Br J Nutr 82, 183–191.
Wijendran V, Hayes KC (2004). Dietary n-6 and n-3 fatty acid balance and cardiovascular health. Annu Rev Nutr 24, 597–615.
Zhao G, Etherton TD, Martin KR, West SG, Gillies PJ, Kris-Etherton PM (2004). Dietary alpha-linolenic acid reduces inflammatory and lipid cardiovascular risk factors in hypercholesterolemic men and women. J Nutr 134, 2991–2997.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guarantors: PLL Goyens and RP Mensink.
Contributors: PLLG and RPM participated intellectually in the development of the paper.
Rights and permissions
About this article
Cite this article
Goyens, P., Mensink, R. Effects of alpha-linolenic acid versus those of EPA/DHA on cardiovascular risk markers in healthy elderly subjects. Eur J Clin Nutr 60, 978–984 (2006). https://doi.org/10.1038/sj.ejcn.1602408
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ejcn.1602408
Keywords
This article is cited by
-
Associations of polyunsaturated fatty acids with cardiovascular disease and mortality: a study of NHANES database in 2003–2018
BMC Endocrine Disorders (2023)
-
Prospective randomized comparison between omega-3 fatty acid supplements plus simvastatin versus simvastatin alone in Korean patients with mixed dyslipidemia: lipoprotein profiles and heart rate variability
European Journal of Clinical Nutrition (2011)
-
Bioavailability of α-linolenic acid from flaxseed diets as a function of the age of the subject
European Journal of Clinical Nutrition (2009)