Abstract
Objective:
The purpose of this study was to determine whether supplements of plant sterols and/or glucomannan improve lipid profile and cholesterol biosynthesis in mildly hypercholesterolemic type II diabetic and non-diabetic subjects and to compare the response of these two subject groups to the treatments.
Design:
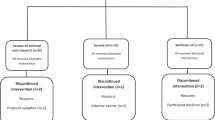
A randomized, crossover study consisting of four phases of 21 days, with each phase separated by a 28-day washout.
Setting:
The Mary Emily Clinical Nutrition Research Unit of McGill University.
Subjects:
Eighteen non-diabetic individuals and 16 type II diabetic individuals aged 38–74 years.
Interventions:
Subjects were supplemented with plant sterols (1.8 g/day), glucomannan (10 g/day), a combination of glucomannan and plant sterols, and a placebo, provided in the form of bars.
Results:
Overall plasma cholesterol concentrations were lowered (P<0.05) after combination treatment (4.72±0.20 mmol/l) compared to control (5.47±0.18 mmol/l). Plasma low-density lipoprotein (LDL) cholesterol concentrations were decreased (P<0.05) after glucomannan (3.16±0.14 mmol/l) and combination treatments (2.95±0.16 mmol/l) compared to control (3.60±0.16 mmol/l). The results of lipid profiles did not differ between subject groups. Overall plasma lathosterol concentrations, an index of cholesterol biosynthesis, were lowered (P<0.05) after the combination treatment compared to the plant sterol treatment.
Conclusions:
The results suggest that glucomannan and a combination of glucomannan and plant sterols substantially improves plasma LDL cholesterol concentrations.
Sponsorship:
Forbes Medi-Tech Inc., Vancouver, British Columbia, Canada.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Arvill A, Bodin L (1995). Effect of short-term ingestion of glucomannan on serum cholesterol in healthy men. Am J Clin Nutr 61, 585–589.
Chen HL, Shen WH, Tai TS, Liaw YP, Chen YC (2003). Konjac supplement alleviated hypercholesterolemia and hyperglycemia in type 2 diabetic subjects – a randomized double-blinded trial. J Am Coll Nutr 22, 36–42.
Doi K (1995). Effects of konjac fiber (glucomannan) on glucose and lipids. Eur J Clin Nutr 49, S190–S197.
Doi K, Masuura M, Kawara A, Baba S (1979). Treatment of diabetes with glucomannan. Lancet 1, 987–988. (abstract).
Doi K, Nakamura T, Aoyama N, Matsuura M, Kawara A (1990). Metabolic and nutritional effects of long-term use of glucomannan in the treatment of obese diabetics. In: Oomura Y, Tarui S, Inoue S and Shimazu T (eds). Progress in Obesity Research 1990. Proceedings of the Sxith International Congress on Obesity. John Libbey: London, pp 507–514.
Expert panel on detection, elevation, and treatment of high blood cholesterol in adults (2001). Executive summary of the third report of the national cholesterol education (NCEP) expert panel on detection, evaluation, and treatment of high cholesterol in adults (adult treatment panel III). JAMA 285, 2486–2497.
Friedewald WT, Levy RI, Fredrickson DS (1972). Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without the use of preparative ultracentrifuge. Clin Chem 18, 499–502.
Gylling H, Miettinen TA (1997). Cholesterol absorption, synthesis, and LDL metabolism in NIDDM. Diabetes Care 20, 90–95.
Gylling H, Puska P, Vartiainen E, Miettinen TA (1999). Serum sterols during stanol ester feeding in a mildly hypercholesterolemic population. J Lipid Res 40, 593–600.
Hallikainen MA, Sarkkinen ES, Gylling H, Erkkila AT, Uusitupa MIJ (2000). Comparison of the effects of plant sterol ester and plant stanol ester-enriched margarines in lowering serum cholesterol concentrations in hypercholesterolaemic subjects on a low fat diet. Eur J Clin Nutr 54, 715–725.
Huang CY, Zhang MY, Peng SS, Hong JR, Wang X, Jiang HJ et al. (1990). Effect of konjac food on blood glucose level in patients with diabetes. Biomed Environ Sci 3, 123–132.
Ikeda I, Tanaka K, Sugano M, Vahouny GV, Gallo LL (1988). Inhibition of cholesterol absorption in rats by plant sterols. J Lipid Res 29, 1573–1582.
Jones PJ, Leitch CA, Pederson RA (1993). Meal-frequency effects on plasma hormone concentrations and cholesterol synthesis in human. Am J Clin Nutr 57, 868–874.
Jones PJ, Reaini-Sarjaz M, Ntanios FY, Vanstone CA, Feng JY, Parsons WD (2000). Modulation of plasma lipids levels and cholesterol kinetics by phytosterol versus phytostanol esters. J Lipid Res 41, 697–705.
Katan MB, Grundy SM, Jones P, Law M, Miettinen T, Paoletti R, Stresa Workshop Participants (2003). Efficacy and safety of plant stanols and sterols in the management of blood cholesterol. Mayo Clin Proc 78, 965–978.
Lakshmanan MR, Nepokroeff CM, Ness GC, Dugan RE, Porter JW (1973). Stimulation of insulin of rat liver 3-hydroxy-3-methylglutaryl coenzyme A reductase and cholesterol synthesis activity. Biochem Biophys Res Commun 50, 704–710.
Matsuura M (1986). Effects of dietary fiber (glucomannan) on serum cholesterol. Jpn Soc Clin Nutr 8, 1–11.
Miettinen TA, Tilvis RS, Kesaniemi YA (1990). Serum plant sterol and cholesterol precursors reflect cholesterol absorption and synthesis in volunteers of a randomly selected male population. Am J Epidemiol 131, 20–31.
Nissinen M, Gylling H, Vuoristo M, Miettinen TA (2002). Micellar distribution of cholesterol and phytosterols after duodenal plant sterol ester infusion. Am J Physiol Gastrointest Liver Physiol 282, G1009–G1015.
Ntanios FY, Jones PJ (1998). Effects of variable dietary sitostanol concentrations on plasma lipid profile and phytosterol metabolism in hamsters. Biochim Biophys Acta 1390, 237–244.
Osborne AR, Pollock VV, Lagor WR, Ness GC (2004). Identification of insulin-responsive region in the HMG-CoA reductase promoter. Biochem Biophys Res Commun 318, 814–818.
Pollack OJ (1953). Reduction of blood cholesterol in man. Circulation 2, 702–706.
Scalfi L, Coltorti A, D'Arrigo E, Carandente V, Mazzacano C, Di Palo M et al. (1987). Effect of dietary fibre on postprandial thermogenesis. Int J Obes Relat Metab Disord 11, 95–99.
Siedel J, Hagele EO, Ziegenhorn J, Wahlefeld AW (1983). Reagent for the enzymatic determination of serum total cholesterol with improved lipolytic efficiency. Clin Chem 29, 1075–1080.
Sugiuchi H, Uji Y, Okabe H, Irie T, Uekama K, Kayahara N et al. (1995). Direct measurement of high-density lipoprotein cholesterol in serum with polyethylene glycol-modified enzymes and sulfated alpha-cyclodextrin. Clin Chem 41, 717–723.
Vanstone CA, Raeini-Sarjaz M, Parsons WE, Jones PJ (2002). Unesterified plant sterols and stanols lower LDL-cholesterol concentrations equivalently in hypercholesterolemic persons. Am J Clin Nutr 76, 1272–1278.
Varady KA, Ebine N, Vanstone CA, Parsons WE, Jones PJ (2004). Plant sterols and endurance training combine to favourably alter plasma lipid profiles in previously sedentary hyporcholesterolemic adults after 8 wk. Am J Clin Nutr 80, 1159–1166.
Vuksan V, Sievenpiper JL, Xu Z, Wong EY, Jenkins AL, Beljan-Zdravkovic U et al. (2001). Konjac-Mannan and American ginsing: emerging alternative therapies for type 2 diabetes mellitus. J Am Coll Nutr 20, 370S–380S.
Vuksan V, Vidgen E, Sievenpiper JL, Brighenti F, Owen R, Josse RG et al. (2000). Beneficial effects of viscous dietary fiber from konjac-mannan in subjects with the insulin resistance syndrome. Diabetes Care 23, 9–14.
Weststrate JA, Meijer GW (1998). Plant sterol-enriched margarines and reduction of plasma total- and LDL-cholesterol concentrations in normocholesterolaemic and mildly hypercholesterolaemic subjects. Eur J Clin Nutr 52, 334–343.
Acknowledgements
We thank Christine Gurekian, Mira Laza and Dr Yanwen Wang for their assistance in conducting the clinical trial, the preparation of the tested bars, and the manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoshida, M., Vanstone, C., Parsons, W. et al. Effect of plant sterols and glucomannan on lipids in individuals with and without type II diabetes. Eur J Clin Nutr 60, 529–537 (2006). https://doi.org/10.1038/sj.ejcn.1602347
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ejcn.1602347
Keywords
This article is cited by
-
Evaluation of chemical components of herbs and spices from Thailand and effect on lipid oxidation of fish during storage
Journal of Food Measurement and Characterization (2023)
-
The effect of Glucomannan on fasting and postprandial blood glucose in adults: a systematic review and meta-analysis of randomized controlled trials
Journal of Diabetes & Metabolic Disorders (2022)
-
Effects of Phytosterols supplementation on blood glucose, glycosylated hemoglobin (HbA1c) and insulin levels in humans: a systematic review and meta-analysis of randomized controlled trials
Journal of Diabetes & Metabolic Disorders (2020)
-
Effect of consuming novel foods consisting high oleic canola oil, barley β-glucan, and DHA on cardiovascular disease risk in humans: the CONFIDENCE (Canola Oil and Fibre with DHA Enhanced) study – protocol for a randomized controlled trial
Trials (2015)
-
Combined Effect of Plant Sterols and Dietary Fiber for the Treatment of Hypercholesterolemia
Plant Foods for Human Nutrition (2014)