Abstract
Objective:
Evaluation of some immune markers in Italian elderly population in relation to zinc status, gender and antioxidant defence.
Design:
Observational study.
Setting:
Italian population.
Subjects:
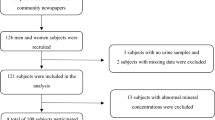
Apparently healthy, free-living subjects, 56 men and 52 women, aged 70–85 y, enrolled in Italy.
Methods:
Lymphocytes were unstimulated or stimulated with the mitogen phytohemoagglutinin (PHA). The proliferative capacity was measured as incorporation of [3H]-thymidine and reported as stimulation index (SI). Cytokine secretion by lymphocytes was determined by ELISA. The antioxidant enzyme activities were measured using commercial kits.
Results:
Dietary zinc intake, as well as zinc in serum, red blood cells and urine were on the normal range of values and did not show any difference between men and women.
Conclusions:
Only weak differences on immune response between men and women were observed. However, zinc status appears to have more influence on the ability of lymphocytes to proliferate in men than in women.
Sponsorship:
The ZENITH study is supported by the European Commission ‘Quality of Life and Management of Living Resources’ Fifth Framework Programme, Contract No: QLK1-CT-2001-00168.
The proliferative response showed a high variability without significant differences between men and women. The amount of secreted pro- and anti-inflammatory cytokines was similar in men and women. No differences were found in the activity of antioxidant enzymes in lymphocytes, namely superoxide dismutase, glutathione peroxidase and catalase, between men and women.
An association between SI and serum zinc level in men was found. SI resulted negatively correlated with interleukin (IL)-1β (R2=0.036 and P=0.012) and IL-10 (R2=0.34 and P=0.040) only in men. IL-10 of PHA-stimulated lymphocytes was negatively correlated with red blood zinc in men (R2=0.41 and P=0.008), while IL-10 of unstimulated and PHA-stimulated lymphocytes were negatively correlated with serum zinc in women (R2=0.38 and P=0.020; R2=0.31 and P=0.040, respectively). No correlation was observed between immune markers and antioxidant enzyme activities.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Ahluwalia N (2004): Aging, nutrition and immune function. J. Nutr. Health Aging 8, 2–61.
Andriollo-Sanchez M, Hininger-Favier I, Meunier N, Toti E, Zaccaria M, Brandollini-Bunlon M, Polito A, O’Connor JM, Ferry M, Coudray C & Roussel AM (2005): Zinc intake and status in middle-aged and older European subjects: the ZENITH study. Eur. J. Clin. Nutr. 59, S37–S41.
Bogden JD (2004): Influence of zinc on immunity in the elderly. J. Nutr. Health Aging 8, 48–54.
Bradford MM (1976): A rapid and sensitive for the quantitation of microgram quantitites of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254.
Chakravarti B & Abraham GN (1999): Aging and T-cell-mediated immunity. Mech. Ageing Dev. 17, 183–206.
Chandra RK (1999): Nutrition and immunology: from the clinic to cellular biology and back again. Proc. Nutr. Soc. 58, 681–683.
Douziech N, Seres I, Larbi A, Szikszay E, Roy PM, Arcand M, Dupuis G & Fulop T (2002): Modulation of human lymphocyte proliferative response with aging. Exp. Gerontol. 37, 369–387.
Fabris N, Mocchegiani E & Provinciali M (1997): Plasticity of neuroendocrine interactions during aging. Exp. Gerontol. 32, 415–429.
Finamore A, Roselli M, Merendino N, Nobili F, Vignolini F & Mengheri E (2003): Zinc deficiency suppresses the development of oral tolerance in rats. J. Nutr. 133, 191–198.
Gaillard RC & Spinedi E (1998): Sex– and stress–steroids interactions and the immune system: evidence for a neuroendocrine–immunological sexual dimorphism. Domest. Anim. Endocrinol. 15, 345–352.
Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE & Roncarolo MG (1997): A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 16, 737–742.
Hirokawa K, Utsuyama M, Katsura Y & Sado T (1988): Influence of age on the proliferation and peripheralization of thymic T cells. Arch. Pathol. Lab. Med. 112, 13–21.
Kraus A, Roth HP & Kirchgessner M (1997): Supplementation with vitamin C, vitamin E or beta-carotene influences osmotic fragility and oxidative damage of erythrocytes of zinc-deficient rats. J. Nutr. 127, 1290–1296.
Levings MK & Roncarolo MG (2005): Phenotypic and functional differences between human CD4+CD25+ and type 1 regulatory T cells. Curr. Top. Microbiol. Immunol. 293, 303–326.
Losonczy G, Kriston T, Szabo A, Muller V, Harvey J, Hamar P, Heeman V & Baylis C (2000): Male gender predisposes to development of endotoxic shock in the rat. Cardiovasc. Res. 47, 183–191.
Malaguarnera L, Ferlito L, Imbesi RM, Gulizia GS, Di Mauro S, Maugeri D, Malaguarnera M & Messina A (2001): Immunosenescence: a review. Arch. Gerontol. Geriatr. 32, 1–14.
McClain CJ, McClain M, Barcve S & Boosalis MG (2002): Trace elements and the elderly. Clin. Geriatr. Med. 18, 801–818.
Offner PJ, Moore EE & Bi WL (1999): Male gender is a risk factor for major infections after surgery. Arch. Surg. Chicago 134, 935–938.
Olsen NJ & Kovacs WJ (1996): Gonadal steroids and immunity. Endocrinol. Rev. 17, 369–384.
Paganelli R, Scala E, Quinti I & Ansotegui IJ (1994): Humoral immunity in aging. Aging (Milano) 6, 143–150.
Polito A, Meunier N, Andriollo-Sanchez M, Catasta G, Azzini E, Simpson EEA, O’Connor JM, Roussel AM, Ferry M, Coudray C & Maiani G (2005): Screening and recruitment procedure of late-middle aged and older European subjects: the ZENITH study. Eur. J. Clin. Nutr. 59, S8–S12.
Prasad AS (1998): Zinc and immunity. Mol. Cell. Biochem. 188, 63–69.
Prasad AS (2000): Effect of zinc deficiency on Th1 and Th2 cytokine shifts. J. Infect. Dis. 182, S62–S68.
Prasad AS, Fitzgerald JT, Hess JW, Kaplan J, Pelen F & Dardenne M (1993): Zinc deficiency in elderly patients. Nutrition 9, 218–224.
Ravaglia G, Forti P, Maioli F, Bastagli L, Facchini A, Mariani E, Savarino L, Sassi S, Cucinotta D & Lenaz G (2000): Effect of micronutrient status on natural killer cell immune function in healthy free-living subjects aged ≥90 y. Am. J. Clin. Nutr. 71, 590–598.
Rink L, Cakman I & Kirchner H (1998): Altered cytokine production in the elderly. Mech. Ageing Dev. 15, 199–209.
Ripa S & Ripa R (1995): Zinc and the elderly. Minerva Med. 86, 275–278.
Sarkar D & Fisher PB (2005): Molecular mechanisms of aging-associated inflammation. Cancer Lett. 21, 1–11.
Sullivan JF, Jetton MM, Hahn HK & Burch RE (1980): Enhanced lipid peroxidation in liver microsomes of zinc-deficient rats. Am. J. Clin. Nutr. 33, 51–56.
Valle B & Falchuk KH (1993): The biochemical basis of zinc. Physiol. Rev. 73, 79–118.
Windmill KF & Lee VM (1998): Effects of castration on the lymphocytes of the thymus, spleen and lymph nodes. Tissue Cell 30, 104–111.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guarantor: E Mengheri.
Contributors: AF performed experiments and data analysis on immune response, CD performed experiments and data analysis on antioxidant enzyme activities, DP contributed to experiments on immune response, MD was responsible for study design and execution of antioxidant experiments, AP was responsible for subjects recruitment and assisted statistical analysis, EV contributed to subjects recruitment and performed erythrocyte preparation, AR performed blood collection, CC was the Zenith study coordinator, EM was responsible for study design, execution and data analysis of immune response experiments and for the preparation of this paper. All authors contributed to finalization of the manuscript.
Rights and permissions
About this article
Cite this article
Finamore, A., Devirgiliis, C., Panno, D. et al. Immune response in relation to zinc status, sex and antioxidant defence in Italian elderly population: the ZENITH study. Eur J Clin Nutr 59 (Suppl 2), S68–S72 (2005). https://doi.org/10.1038/sj.ejcn.1602302
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ejcn.1602302
Keywords
This article is cited by
-
The immune system and the impact of zinc during aging
Immunity & Ageing (2009)
-
Introduction to the ZENITH study and summary of baseline results
European Journal of Clinical Nutrition (2005)