Abstract
Objective:
To investigate the effects of cold storage and vinegar addition on glycaemic and insulinaemic responses to a potato meal in healthy subjects.
Subjects and setting:
A total of 13 healthy subjects volunteered for the study, and the tests were performed at Applied Nutrition and Food Chemistry, Lund University, Sweden.
Experimental design and test meals:
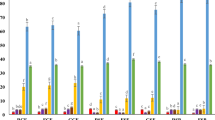
The study included four meals; freshly boiled potatoes, boiled and cold stored potatoes (8°C, 24 h), boiled and cold stored potatoes (8°C, 24 h) with addition of vinaigrette sauce (8 g olive oil and 28 g white vinegar (6% acetic acid)) and white wheat bread as reference. All meals contained 50 g available carbohydrates and were served as a breakfast in random order after an overnight fast. Capillary blood samples were collected at time intervals during 120 min for analysis of blood glucose and serum insulin. Glycaemic (GI) and insulinaemic indices (II) were calculated from the incremental areas using white bread as reference.
Results:
Cold storage of boiled potatoes increased resistant starch (RS) content significantly from 3.3 to 5.2% (starch basis). GI and II of cold potatoes added with vinegar (GI/II=96/128) were significantly reduced by 43 and 31%, respectively, compared with GI/II of freshly boiled potatoes (168/185). Furthermore, cold storage per se lowered II with 28% compared with the corresponding value for freshly boiled potatoes.
Conclusion:
Cold storage of boiled potatoes generated appreciable amounts of RS. Cold storage and addition of vinegar reduced acute glycaemia and insulinaemia in healthy subjects after a potato meal. The results show that the high glycaemic and insulinaemic features commonly associated with potato meals can be reduced by use of vinegar dressing and/or by serving cold potato products.
Sponsorship:
The Swedish Agency for Innovation Systems (Project No P11900-3 A) and Öresund Starch Profiles (ÖSP).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Asp NG (1992): Resistant starch – Proceedings from the 2nd Plenary Meeting of Euresta – European Flair Concerted Action 11 on Physiological Implications of the Consumption of Resistant Starch in Man. Eur. J. Clin. Nutr. 46, S1.
Berry CS (1986): Resistant starch: formation and measurement of starch that survives exhaustive digestion with amylolytic enzymes during the determination of dietary fibre. J. Cereal Sci. 4, 301–314.
Björck I, Liljeberg H & Östman E (2000): Low glycaemic-index foods. Br. J. Nutr. 83, S149–S155.
Björck IM & Siljeström MA (1992): In-vivo and in-vitro digestibility of starch in autoclaved pea and potato products. J. Sci. Food Agric. 58, 541–553.
Brighenti F, Castellani G, Benini L, Casiraghi MC, Leopardi E, Crovetti R & Testolin G (1995): Effect of neutralized and native vinegar on blood glucose and acetate responses to a mixed meal in healthy subjects. Eur. J. Clin. Nutr. 49, 242–247.
Brouns F, Kettlitz B & Arrigoni E (2002): Resistant starch and ‘the butyrate revolution’. Trends Food Sci. Technol. 13, 251–261.
Cummings JH, Beatty ER, Kingman SM, Bingham SA & Englyst HN (1996): Digestion and physiological properties of resistant starch in the human large bowel. Br. J. Nutr. 75, 733–747.
Dysseler P & Hoffem D (1994): Estimation of resistant starch intake in Europe. In Proceedings of the concluding plenary meeting of EURESTA, April 1994. European Flair – Concerted Action no. 11 (COST 911) eds N-G Asp, JMM van Amelsvoort, JGAJ Hautvast, Wageningen: European Commission.
Ebihara K & Nakajima A (1988): Effect of acetic-acid and vinegar on blood-glucose and insulin responses to orally-administered sucrose and starch. Agric. Biol. Chem. 52, 1311–1312.
Englyst HN & Cummings JH (1987): Digestion of polysaccharides of potato in the small intestine of man. Am. J. Clin. Nutr. 45, 423–431.
Foster-Powell K, Holt SH & Brand-Miller JC (2002): International table of glycemic index and glycemic load values: 2002. Am. J. Clin. Nutr. 76, 5–56.
Fredriksson H, Björck I, Andersson R, Liljeberg H, Silverio J, Eliasson A-C & Åman P (2000): Studies on α-amylase degradation of retrograded starch gels from waxy maize and high-amylopection potato. Carbohydr. Polym. 43, 81–87.
Fredriksson H, Silverio J, Andersson R, Eliasson A-C & Åman P (1998): The influence of amylose and amylopectin characteristics on gelatinization and retrogradation properties of different starches. Carbohydr. Polym. 35, 119–134.
Fushimi T, Tayama K, Fukaya M, Kitakoshi K, Nakai N, Tsukamoto Y & Sato Y (2001): Acetic acid feeding enhances glycogen repletion in liver and skeletal muscle of rats. J. Nutr. 131, 1973–1977.
Garcia-Alonso A & Goni I (2000): Effect of processing on potato starch: in vitro availability and glycaemic index. Nahrung. 44, 19–22.
Granfeldt Y, Drews A & Björck I (1995): Arepas made from high amylose corn flour produce favorably low glucose and insulin responses in healthy humans. J. Nutr. 125, 459–465.
Hallert C, Björck I, Nyman M, Pousette A, Granno C & Svensson H (2003): Increasing fecal butyrate in ulcerative colitis patients by diet: controlled pilot study. Inflamm. Bowel Dis. 9, 116–121.
Holm J, Björck I, Drews A & Asp N-G (1986): A rapid method for the analysis of starch. Starch/Stärke 38, 224–226.
Jenkins DJ, Jenkins AL, Wolever TM, Collier GR, Rao AV & Thompson LU (1987): Starchy foods and fiber: reduced rate of digestion and improved carbohydrate metabolism. Scand. J. Gastroenterol. Suppl. 129, 132–141.
Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM, Bowling AC, Newman HC, Jenkins AL & Goff DV (1981): Glycemic index of foods: a physiological basis for carbohydrate exchange. Am. J. Clin. Nutr. 34, 362–366.
Johnston CS, Kim CM & Buller AJ (2004): Vinegar improves insulin sensitivity to a high-carbohydrate meal in subjects with insulin resistance or type 2 diabetes. Diabetes Care 27, 281–282.
Kanan W, Bijlani RL, Sachdeva U, Mahapatra SC, Shah P & Karmarkar MG (1998): Glycaemic and insulinaemic responses to natural foods, frozen foods and their laboratory equivalents. Indian J. Physiol. Pharmacol. 42, 81–89.
Kingman SM & Englyst HN (1994): The influence of food preparation methods on the in-vitro digestibility of starch in potatoes. Food Chem. 49, 181–186.
Liljeberg H & Björck I (1994): Bioavailability of starch in bread products. Postprandial glucose and insulin responses in healthy subjects and in vitro resistant starch content. Eur. J. Clin. Nutr. 48, 151–163.
Liljeberg H & Björck I (1998): Delayed gastric emptying rate may explain improved glycaemia in healthy subjects to a starchy meal with added vinegar. Eur. J. Clin. Nutr. 52, 368–371.
Liljeberg HG, Lönner CH & Björck IM (1995): Sourdough fermentation or addition of organic acids or corresponding salts to bread improves nutritional properties of starch in healthy humans. J. Nutr. 125, 1503–1511.
Liu S, Willett WC, Stampfer MJ, Hu FB, Franz M, Sampson L, Hennekens CH & Manson JE (2000): A prospective study of dietary glycemic load, carbohydrate intake, and risk of coronary heart disease in US women. Am. J. Clin. Nutr. 71, 1455–1461.
Martin LJM, Dumon HJW & Champ MMJ (1998): Production of short-chain fatty acids from resistant starch in a pig model. J. Sci. Food Agric. 77, 71–80.
Matsuda M & DeFronzo RA (1999): Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22, 1462–1470.
McKeown NM, Meigs JB, Liu S, Saltzman E, Wilson PW & Jacques PF (2004): Carbohydrate nutrition, insulin resistance, and the prevalence of the metabolic syndrome in the Framingham Offspring Cohort. Diabetes Care 27, 538–546.
Ogawa N, Satsu H, Watanabe H, Fukaya M, Tsukamoto Y, Miyamoto Y & Shimizu M (2000): Acetic acid suppresses the increase in disaccharidase activity that occurs during culture of caco-2 cells. J. Nutr. 130, 507–513.
Raben A, Tagliabue A, Christensen NJ, Madsen J, Holst JJ & Astrup A (1994): Resistant starch: the effect on postprandial glycemia, hormonal response, and satiety. Am. J. Clin. Nutr. 60, 544–551.
Roediger WE (1980): Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut 21, 793–798.
Salmeron J, Ascherio A, Rimm EB, Colditz GA, Spiegelman D, Jenkins DJ, Stampfer MJ, Wing AL & Willett WC (1997a): Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care 20, 545–550.
Salmeron J, Manson JE, Stampfer MJ, Colditz GA, Wing AL & Willett WC (1997b): Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. J. Am. Med. Assoc. 277, 472–477.
Scheppach W, Fabian C, Sachs M & Kasper H (1988): The effect of starch malabsorption on fecal short-chain fatty acid excretion in man. Scand. J. Gastroenterol. 23, 755–759.
Soh NL & Brand-Miller J (1999): The glycaemic index of potatoes: the effect of variety, cooking method and maturity. Eur. J. Clin. Nutr. 53, 249–254.
Sugiyama M, Tang AC, Wakaki Y & Koyama W (2003): Glycemic index of single and mixed meal foods among common Japanese foods with white rice as a reference food. Eur. J. Clin. Nutr. 57, 743–752.
Wolever TM (1990): The glycemic index. World Rev. Nutr. Diet. 62, 120–185.
Åkerberg AK, Liljeberg HG, Granfeldt YE, Drews AW & Björck IM (1998): An in vitro method, based on chewing, to predict resistant starch content in foods allows parallel determination of potentially available starch and dietary fiber. J. Nutr. 128, 651–660.
Östman E, Granfeldt Y, Persson L & Björck I (2005): Vinegar supplementation lowers glucose and insulin responses and increases satiety after a bread meal in healthy subjects. The doi-number is: 10.1038/sj.ejcn.1602197.
Östman EM, Liljeberg Elmståhl HG & Björck IM (2002a): Barley bread containing lactic acid improves glucose tolerance at a subsequent meal in healthy men and women. J. Nutr. 132, 1173–1175.
Östman EM, Nilsson M, Liljeberg Elmståhl H, Molin G & Björck I (2002b): On the effect of lactic acid on blood glucose and insulin responses to cereal products: mechanistic studies in healthy subjects and in vitro. J. Cereal Sci. 36, 339–346.
Acknowledgements
We thank Lisbeth Persson for invaluable technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guarantor: I Björck.
Contributors: ML, EÖ and IB made the design of the study. ML was responsible for collection and analysis of data. ML, EÖ and IB contributed to the writing of the manuscript.
Rights and permissions
About this article
Cite this article
Leeman, M., Östman, E. & Björck, I. Vinegar dressing and cold storage of potatoes lowers postprandial glycaemic and insulinaemic responses in healthy subjects. Eur J Clin Nutr 59, 1266–1271 (2005). https://doi.org/10.1038/sj.ejcn.1602238
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ejcn.1602238
Keywords
This article is cited by
-
Study on nutritional quality and volatile aroma compounds of the stir-fried shredded potatoes
American Journal of Potato Research (2022)
-
Glycemic response, satiety, gastric secretions and emptying after bread consumption with water, tea or lemon juice: a randomized crossover intervention using MRI
European Journal of Nutrition (2022)
-
The cumulative effects of chilling and reheating a carbohydrate-based pasta meal on the postprandial glycaemic response: a pilot study
European Journal of Clinical Nutrition (2021)
-
Lemon juice, but not tea, reduces the glycemic response to bread in healthy volunteers: a randomized crossover trial
European Journal of Nutrition (2021)
-
Application of Organic Acid Based Artificial Neural Network Modeling for Assessment of Commercial Vinegar Authenticity
Food Analytical Methods (2016)