Abstract
Objective:
The effect of drinking deep-sea water on hair minerals was studied in patients with atopic eczema/dermatitis syndrome (AEDS). Study of hair minerals revealed an imbalance of essential minerals and an increase in toxic minerals in AEDS patients.
Design:
After drinking deep-sea water (Amami no Mizu) for 6 months in AEDS patients, hair minerals (essential minerals and toxic minerals), clinical evaluation of the skin symptoms were compared before drinking with after drinking.
Subjects:
After obtaining informed consent, 33 patients (mean age 26 y, range 1–50 y, 13 male and 20 female subjects) with mild to moderate AEDS were enrolled.
Results:
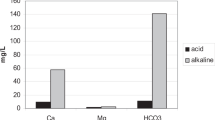
After drinking deep-sea water, the levels of the essential mineral, potassium (K), were significantly decreased, while the levels of selenium (Se) increased. On the other hand, drinking deep-sea water significantly decreased the levels of the toxic minerals, mercury and lead. Moreover, after drinking deep-sea water, the skin symptoms were improved in 27 out of 33 patients.
Conclusion:
These results indicate that the mineral abnormalities/imbalance may be involved in the pathogenesis of AEDS, and that drinking deep-sea water may be useful in the treatment of AEDS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
David TJ, Wella FE, Sharpe TC & Gibbs ACC (1984): Low serum zinc in children with atopic eczema. Br. J. Dermatol. 111, 597–601.
Denda M, Hosoi J & Asida Y (2000): Visual imaging of ion distribution in human epidermis. Biochem. Biophys. Res. Commun. 272, 134–137.
Denda M & Kumazawa N (2002): Negative electric potential induces alteration of ion gradient and lamellar body secretion in the epidermis, and accelerates skin barrier recovery after barrier disruption. J. Invest. Dermatol. 118, 65–72.
Edelberg R (1977): Relation of electrical properties of skin to structure and physiologic state. J. Invest. Dermatol. 69, 324–327.
Emelyanov A, Fedoseev G & Barnes PJ (1999): Reduced intracellular magnesium concentrations in asthmatic patients. Eur. Respir. J. 13, 38–40.
Hanifin JM & Rajka G (1980): Diagnostic features of atopic dermatitis. Acta Dermatovene (Stockholm) 92 (Suppl), 44–47.
Ito Y, Nagai N, Syumiya M, Inomata K, Tai H, Hataguchi Y, Nakagawa K & Nakajima H (2003): Influence of the magnesium and the calcium content in high mineral water adjusted from deep sea water affect the cataract delay effect of a hereditary cataract rat SCR. The 42th Japanese Society for Cataract Research 126 (In Japanese).
Kimata H (2001): Increased SPT reactions in fatty liver. Allergy 56, 798–799.
Kimata H, Tai H & Nakajima H (2001): Reduction of allergic skin responses and serum allergen-specific IgE and IgE-inducing cytokines by drinking deep-sea water in patients with allergic rhinitis. Otorhinolaryngol Nova 11, 302–303.
Kimata H, Tai H, Nakagawa K, Yokoyama Y, Nakajima H & Ikegami Y (2002): Improvement of skin symptoms and mineral imbalance by drinking deep sea water in patients with atopic eczema/dermatitis syndrome. Acta Medica (Hradec Kralove) 45, 83–84.
Makiura M, Akamatsu H, Akita H, Yagami A, Shimizu Y, Eiro H, Kuramoto M, Suzuki K & Matsunaga K (2004): Atopic dermatitis-like symptoms in HR-1 hairless mice fed a diet low in magnesium and zinc. J. Int. Med. Res. 32, 392–399.
Neckermann G, Bavandi A & Meingassner JG (2000a): Atopic dermatitis-like symptoms in hypomagnesaimic hairless rats and inhibited by systemic or topical SDS ASW 981. Br. J. Dermatol. 142, 669–679.
Neckermann G, Bavandi A & Meingassner JG (2000b): Atopic dermatitis-like symptoms in hypomagnesaemic hairless rats are prevented and inhibited by systemic or topical SDZ ASM 981. Br. J. Dermatol. 142, 669–679.
Niwa Y & Iizawa O (1994): Abnormalities in serum lipids and leukocyte superoxide dismutase and associated cataract formation in patients with atopic dermatitis. Arch. Dermatol. 130, 1387–1392.
Niwa Y, Sumi H, Kawahira K, Terashimsa T, T & Akamatsu H (2003): Protein oxidative damage in the stratum corneum: evidence for a link between environmental oxidants and the changing prevalence and nature of atopic dermatitis in Japan. Br. J. Dermatol. 149, 248–254.
Sanna E, Liguroi A, Palmas L, Soro MR & Floris G (2003): Blood and hair lead in boys and girls living in two Sardinian towns at different risks of lead pollution. Ecotoxicol. Environ. Saf. 55, 293–299.
Staquet MJ, Peguet-Navarro J, Richard A, Schmitt D & Rougier A (2002): In vitro effect of a spa water on the migratory and stimulatory capacities on human Langerhans cells. Eur. J. Dermatol. 12, 59–61 (LIX-LXI).
Tabary O, Muselet C, Yvin JC, Halley-Vanhove B, Puchelle E & Jacquot J (2001): Physiomer® reduces the chemokine interleukin-8 production by activated human respiratoty epithelial cells. Eur. Respir. J. 18, 661–666.
Ukabam SO, Mann RJ & Cooper BT (1984): Small intestinal permeability to sugars in patients with atopic eczema. Br. J. Dermatol. 110, 649–652.
Wilhelm M, Passlick J, Busch T, Szydlik M & Ohnesorge FK (1989): Scalp hair as an indicator of aluminium exposure: comparison to bone and plasma. Hum. Toxicol. 8, 5–9.
Acknowledgements
We are indebted to Mr Toyoharu Tsutsui (LVB Preventive Medicine Laboratory) for his kind analysis of the hair minerals and helpful discussions. We wish to express our gratitude to Dr Jun Yoshizawa (Yoshizawa Clinic of Dermatology), Dr Misa Sasai (Kansai Medical University), Dr Yoko Taniguchi (Taniguchi Clinic), Dr Sachie Sumida (Sachie-hihuka Clinic), and Dr Atsuko Ymakami (Matsusaka Chuo General Hospital).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hataguchi, Y., Tai, H., Nakajima, H. et al. Drinking deep-sea water restores mineral imbalance in atopic eczema/dermatitis syndrome. Eur J Clin Nutr 59, 1093–1096 (2005). https://doi.org/10.1038/sj.ejcn.1602218
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ejcn.1602218
Keywords
This article is cited by
-
Mineral-balanced deep sea water enhances the inhibitory effects of chitosan oligosaccharide on atopic dermatitis-like inflammatory response
Biotechnology and Bioprocess Engineering (2017)
-
Stimulatory Effect of Balanced Deep-Sea Water Containing Chitosan Oligosaccharides on Glucose Uptake in C2C12 Myotubes
Marine Biotechnology (2016)
-
Effect of the salts of deep ocean water on the production of cordycepin and adenosine of Cordyceps militaris-fermented product
AMB Express (2015)
-
The advantages of deep ocean water for the development of functional fermentation food
Applied Microbiology and Biotechnology (2015)
-
Deep-sea water inhibits metastatic potential in HT-29 human colorectal adenocarcinomas via MAPK/NF-κB signaling pathway
Biotechnology and Bioprocess Engineering (2014)