Abstract
Objective: To study the effects of 13 weeks conjugated linoleic acid (CLA) supplementation in overweight subjects on body-weight maintenance, parameters of appetite and energy intake (EI) at breakfast after weight loss.
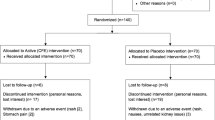
Design: This study had a double-blind, placebo-controlled randomized design.
Subjects: A total of 26 men and 28 women (age 37.8±7.7 y; body mass index 27.8±1.5 kg/m2).
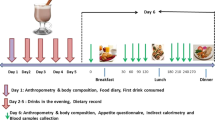
Interventions: Subjects were first submitted to a very-low-calorie diet (VLCD; 2.1 MJ/day) for 3 weeks after which they started with the 13-week intervention period. They either received 1.8 g CLA or placebo per day or 3.6 g CLA or placebo per day. Additionally, subjects of the high dosage intervention replaced their habitual lunch by one meal of a protein-rich, low-energy supplement. EI was measured at breakfast and appetite profile after an overnight fast.
Results: The mean body weight loss was 6.9±1.7% of their original body weight. Multiple regression analysis showed that at the end of the 13-week intervention, CLA did not have an effect on body weight regain. Feelings of fullness and satiety were increased and feelings of hunger were decreased after 13 weeks intervention by CLA compared to placebo, independent of %body weight regain. However, EI measured at breakfast was not affected by CLA.
Conclusions: Appetite (hunger, satiety and fullness) was favorably, dose-independently affected by a 13-week consumption of 1.8 or 3.6 g CLA/day. This did not result in a lower EI at breakfast or an improved body-weight maintenance after weight loss.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ashley JM, St Jeor ST, Perumean-Chaney S, Schrage J & Bovee V (2001): Meal replacements in weight intervention. Obes. Res. 9(Suppl 4), 312S–320S.
Azain MJ, Hausman DB, Sisk MB, Flatt WP & Jewell DE (2000): Dietary conjugated linoleic acid reduces rat adipose tissue cell size rather than cell number. J. Nutr. 130, 1548–1554.
Berven G, Bye A, Hals O, Blankson H, Fagertun H, Thom E, Wadstein J & Gudmundsen O (2000): Safety of conjugated linoleic acid (CLA) in overweight and obese human volunteers. Eur. J. Lipid Sci. Technol. 102, 155–462.
Blankson H, Stakkestad JA, Fagertun H, Thom E, Wadstein J & Gudmundsen O (2000): Conjugated linoleic acid reduces body fat mass in overweight and obese humans. J. Nutr. 130, 2943–2948.
Choi Y, Kim YC, Han YB, Park Y, Pariza MW & Ntambi JM (2000): The trans-10, cis-12 isomer of conjugated linoleic acid downregulates stearoyl-CoA desaturase 1 gene expression in 3T3-L1 adipocytes. J. Nutr. 130, 1920–1924.
DeLany JP, Blohm F, Truett AA, Scimeca JA & West DB (1999): Conjugated linoleic acid rapidly reduces body fat content in mice without affecting energy intake. Am. J. Physiol. 276, R1172–R1179.
Ens JG, Ma DW, Cole KS, Field CJ & Clandinin MT (2001): An assessment of c9,t11 linoleic acid intake in a small group of young Canadians. Nutr. Res. 21, 955–960.
Flatt JP (1996): Glycogen levels and obesity. Int J Obes Relat Metab Disord 20(Suppl 2), S1–S11.
Halvorsen YD, Lea-Currie R, Geigerman C & McIntosh M (2000): Conjugated linoleic acid (CLA) attenuates human preadipocyte triglyceride (TG) content and lipogenesis. In NAASO Meeting; Long Beach, USA. Obesity Research, S121.
Herman CP & Polivy J (1980): Restrained eating. In Obesity, ed. AJ Stunkard, ed. pp 208–225. Philadelphia: WB Saunders.
Kepler CR, Tucker WP & Tove SB (1970): Biohydrogenation of unsaturated fatty acids. IV. Substrate specificity and inhibition of linoleate delta-12-cis, delta-11-trans-isomerase from Butyrivibrio fibrisolvens. J. Biol. Chem. 245, 3612–3620.
Kepler CR, Tucker WP & Tove SB (1971): Biohydrogenation of unsaturated fatty acids. V. Stereospecificity of proton addition and mechanism of action of linoleic acid delta 12-cis, delta 11-trans-isomerase from Butyrivibrio fibrisolvens. J. Biol. Chem. 246, 2765–2771.
Mattes RD (1990): Hunger ratings are not a valid proxy measure of reported food intake in humans. Appetite 15, 103–113.
Medina EA, Horn WF, Keim NL, Havel PJ, Benito P, Kelley DS, Nelson GJ & Erickson KL (2000): Conjugated linoleic acid supplementation in humans: effects on circulating leptin concentrations and appetite. Lipids 35, 783–788.
Melanson KJ, Westerterp-Plantenga MS, Campfield LA & Saris WHM (1999): Appetite and blood glucose profiles in humans after glycogen-depleting exercise. J. Appl. Physiol. 87, 947–954.
Miner JL, Cederberg CA, Nielsen MK, Chen X & Baile CA (2001): Conjugated linoleic acid (CLA), body fat, and apoptosis. Obes. Res. 9, 129–134.
Ohnuki K, Haramizu S, Oki K, Ishihara K & Fushiki T (2001): A single oral administration of conjugated linoleic acid enhanced energy metabolism in mice. Lipids 36, 583–587.
Pariza MW, Park Y & Cook ME (2001): The biologically active isomers of conjugated linoleic acid. Prog. Lipid Res. 40, 283–298.
Park Y, Albright KJ, Liu W, Storkson JM, Cook ME & Pariza MW (1997): Effect of conjugated linoleic acid on body composition in mice. Lipids 32, 853–858.
Park Y, Storkson JM, Albright KJ, Liu W & Pariza MW (1999a): Evidence that the trans-10,cis-12 isomer of conjugated linoleic acid induces body composition changes in mice. Lipids 34, 235–241.
Park Y, Albright KJ, Storkson JM, Liu W, Cook ME & Pariza MW (1999b): Changes in body composition in mice during feeding and withdrawal of conjugated linoleic acid. Lipids 34, 243–248.
Risérus U, Berglund L & Vessby B (2001): Conjugated linoleic acid (CLA) reduced abdominal adipose tissue in obese middle-aged men with signs of the metabolic syndrome: a randomised controlled trial. Int. J. Obes. Relat. Metab. Disord. 25, 1129–1135.
Sisk MB, Hausman DB, Martin RJ & Azain MJ (2001): Dietary conjugated linoleic acid reduces adiposity in lean but not obese Zucker rats. J. Nutr. 131, 1668–1674.
Stock MJ (1999): Gluttony and thermogenesis revisited. Int. J. Obes. Relat. Metab. Disord. 23: 1105–1107.
Stunkard AJ & Messick S (1985): The three-factor eating questionnaire to measure dietary restraint, disinhibition, and hunger. J. Psychosom. Res. 29, 71–83.
Terpstra AH (2001): Differences between humans and mice in efficacy of the body fat lowering effect of conjugated linoleic acid: role of metabolic rate. J. Nutr. 131, 2067–2068.
West DB, Blohm FY, Truett AA & DeLany JP (2000): Conjugated linoleic acid persistently increases total energy expenditure in AKR/J mice without increasing uncoupling protein gene expression. J. Nutr. 130, 2471–2477.
West DB, Delany JP, Camet PM, Blohm F, Truett AA & Scimeca J (1998): Effects of conjugated linoleic acid on body fat and energy metabolism in the mouse. Am. J. Physiol. 275, R667–R672.
Westerterp-Plantenga MS & Verwegen CRT (1999): The appetizing effect of an aperitif in overweight and normal-weight humans. Am. J. Clin. Nutr. 69, 205–212.
Westerterp-Plantenga MS (2000): Eating behavior in humans, characterized by cumulative food intake curves—a review. Neurosci. Biobehav. Rev. 24, 239–248.
Westerterp-Plantenga MS & Kovacs EM (2002): The effect of (-)-hydroxycitrate on energy intake and satiety in overweight humans. Int. J. Obes. Relat. Metab. Disord. 26, 870–872.
Westerterp-Plantenga MS, Saris WH, Hukshorn CJ & Campfield LA (2001): Effects of weekly administration of pegylated recombinant human OB protein on appetite profile and energy metabolism in obese men. Am. J. Clin. Nutr. 74, 426–434.
Zambell KL, Keim NL, Van Loan MD, Gale B, Benito P, Kelley DS & Nelson GJ (2000): Conjugated linoleic acid supplementation in humans: effects on body composition and energy expenditure. Lipids 35, 777–782.
Acknowledgements
This study was supported by Novartis Consumer Health Ltd, Nyon, Switzerland. We thank Hovdebygda, Norway, for providing the CLA and placebo capsules, and Winyee To for her assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kamphuis, M., Lejeune, M., Saris, W. et al. Effect of conjugated linoleic acid supplementation after weight loss on appetite and food intake in overweight subjects. Eur J Clin Nutr 57, 1268–1274 (2003). https://doi.org/10.1038/sj.ejcn.1601684
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ejcn.1601684
Keywords
This article is cited by
-
Dietary conjugated linoleic acid and medium-chain triglycerides for obesity management
Journal of Biosciences (2021)
-
The effects of whole milk compared to skim milk and apple juice consumption in breakfast on appetite and energy intake in obese children: a three-way randomized crossover clinical trial
BMC Nutrition (2018)
-
Effects of low-fat milk consumption at breakfast on satiety and short-term energy intake in 10- to 12-year-old obese boys
European Journal of Nutrition (2016)
-
A Review of the Evidence Supporting the Taste of Non‐esterified Fatty Acids in Humans
Journal of the American Oil Chemists' Society (2016)
-
Pros and cons of CLA consumption: an insight from clinical evidences
Nutrition & Metabolism (2015)