Abstract
Objective: To compare the effect of a modified fat, monounsaturated-fat-enriched diet and a high-carbohydrate low-fat diet with high lycopene content on the serum concentration of lycopene and other carotenoids.
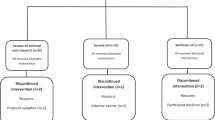
Design: A randomised crossover dietary intervention study.
Setting:Melbourne, Australia — Healthy free-living men.
Subjects: A total of 13 healthy males between the age of 20 and 70 y, recruited via advertisements in newspapers and university newsletter.
Intervention: A randomised dietary intervention with two diets of 14 days each. The two diets were — (1) high-fat monounsaturated-fat-enriched (MUFA) and (2) high-carbohydrate low-fat (HCLF). Both the diets contained the same basic foods and a controlled carotenoid content high in lycopene.
Results: A significant increase in serum total lycopene occurred, by 126% on the MUFA diet (P<0.001) and 108% on the HCLF diet (P=0.001). A reduction in serum cryptoxanthin (27% on MUFA diet and 25% on HCLF) and alpha-carotene (43% on the MUFA diet and 25% on the HCLF diet) was observed. No change was observed for the other carotenoids. Comparing the end of the two diets, no statistically significant difference was observed for lycopene or the other carotenoids.
Conclusion: In all, 15% of energy from fat or 38% of energy from fat (predominantly monounsaturated fat) in the diet does not have a significant differential effect on serum lycopene.
Sponsorship: The study was partially funded by the Grains Research Development Corporation, Canberra and Meadow Lea Foods Ltd, Mascot, Australia. HJ Heinz, Melbourne, Australia provided the tomato products and some funds for their carotenoid analysis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Ashton EL, Best JD &, Ball MJ (2001): Effects of monounsaturated enriched sunflower oil on CHD risk factors including LDL size and copper-induced LDL oxidation. J. Am. Coll. Nutr. 20, 320–326.
ATBC (1994): The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N. Engl. J. Med. 330, 1029–1035.
Berry EM, Eisenberg S, Friedlander Y, Harats D, Kauftnann NA, Norman Y &, Stein Y (1992): Effects of diets rich in monounsaturated fatty acids on plasma lipoproteins—the Jerusalem Nutrition Study. II. Monounsaturated fatty acids vs carbohydrates. Am. J. Clin. Nutr. 56, 394–403.
Bingham SA (1991): Limitations of the various methods for collecting dietary intake data. Ann. Nutr. Metab. 35, 117–127.
Block G (1982): A review of validations of dietary assessment methods. Am. J. Epidemiol. 115, 492–505.
Bowen PE, Garg V, Stacewicz Sapuntzakis M, Yelton L &, Schreiner RS (1993): Variability of serum carotenoids in response to controlled diets containing six servings of fruits and vegetables per day. Ann. N Y Acad. Sci. 691, 241–243.
Chasan-Taber L, Willett WC, Seddon JM, Stampfer MJ, Rosner B, Colditz GA, Speizer FE &, Hankinson SE (1999): A prospective study of carotenoid and vitamin A intakes and risk of cataract extraction in US women. Am. J. Clin. Nutr. 70, 509–516.
Clarke R (1997): Dietary lipids and blood cholesterol: quantitative meta-analysis of metabolic ward studies. BMJ 314, 112–117.
de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J &, Mamelle N (1999): Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation 99, 779–785.
Despres JP, Lemieux I, Dagenais GR, Cantin B &, Lamarche B (2000): HDL-cholesterol as a marker of coronary heart disease risk: the Quebec cardiovascular study. Atheroselerosis 153, 263–272.
Di Mascio P, Kaiser S &, Sies H (1989): Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch. Biochem. Biophys. 274, 532–538.
Dimitrov NV, Meyer C, Ullrey DE, Chenoweth W, Michelakis A, Malone W, Boone C &, Fink G (1988): Bioavailability of beta-carotene in humans. Am. J. Clin. Nutr. 48, 298–304.
D'Odorico A, Martines D, Kiechl S, Egger G, Oberhollenzer F, Bonvicini P, Sturniolo GC, Naccarato R &, Willeit J (2000): High plasma levels of alpha- and beta-carotene are associated with a lower risk of atherosclerosis: results from the Bruneck study. Atherosclerosis 153, 231–239.
Elinder LS, Hadell K, Johansson L, Holme JM, Olsson AG &, Walldius G (1995): Probucol treatment decreases serum concentration of diet-derived antioxidants. Arterioscler. Thromb. Vasc. Biol. 15, 1057–1631.
Forman MR, Beecher GR, Lanza E, Reichman ME, Graubard BI, Campbell WS, Marr T, Yong LC, Judd JT &, Taylor PR (1995): Effect of alcohol consumption on plasma carotenoid concentrations in premenopausal women: a controlled dietary study. Am. J. Clin. Nutr. 62, 131–135.
Friedewald WT, Levy RI &, Fredrickson DS (1972): Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18, 499–502.
Fuhrman B, Elis A &, Aviram M (1997): Hypocholesterolemic effect of lycopene and beta-carotene is related to suppression of cholesterol synthesis and augmentation of LDL receptor activity in macrophages. Biochem. Biophys. Res. Commun. 233, 658–662.
Garg A, Bonanome A, Grundy SM, Zhang ZJ &, Unger RH (1988): Comparison of a high-carbohydrate diet with a high-monoun-saturated-fat diet in patients with noninsulin-dependent diabetes mellitus. N. Engl. J. Med. 319, 829–834.
Giovannucci E, Ascherio A, Rimm EB, Stampfer ML, Colditz GA &, Willett WC (1995): Intake of carotenoids and retinol in relation to risk of prostate cancer. J. Natl. Cancer Inst. 87, 1767–1776.
Grundy SM, Florentin L, Nix D &, Whelan MF (1988): Comparison of monounsaturated fatty acids and carbohydrates for reducing raised levels of plasma cholesterol in man. Am. J. Clin. Nutr. 47, 965–969.
Haskell WL, Alderman EL, Fair JM, Maron DJ, Mackey SF, Superko HR, Williams PT, Johnstone IM, Champagne MA &, Krauss RM (1994): Effects of intensive multiple risk factor reduction on coronary atherosclerosis and clinical cardiac events in men and women with coronary artery disease. The Stanford Coronary Risk Intervention Project (SCRIP). Circulation 89, 975–990.
Hennekens CH, Buring JE, Manson JE, Stampfer M, Rosner B, Cook NR, Belanger C, LaMotte F, Gaziano JM, Ridker PM, Willett W &, Peto R (1996): Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N. Engl. J. Med. 334, 1145–1149.
Jacques PF &, Chylack Jr LT (1991): Epidemiologic evidence of a role for the antioxidant vitamins and carotenoids in cataract prevention. Am. J. Clin. Nutr. 53, 352S–355S.
Jalal F, Nesheim MC, Agus Z, Sanjur D &, Habicht JP (1998): Serum retinol concentrations in children are affected by food sources of beta-carotene, fat intake, and anthelmintic drug treatment. Am. J. Clin. Nutr. 68, 623–629.
Jayarajan P, Reddy V &, Mohanram M (1980): Effect of dietary fat on absorption of beta carotene from green leafy vegetables in children. Indian J. Med. Res. 71, 53–56.
Johnson EJ, Qin J, Krinsky NI &, Russell RM (1997): Ingestion by men of a combined dose of beta-carotene and lycopene does not affect the absorption of beta-carotene but improves that of lycopene. J. Nutr. 127, 1833–1837.
Katan MB, Grundy SM &, Willett WC (1997): Should a low-fat, high-carbohydrate diet be recommended for everyone? Beyond low-fat diets. N. Engl. J. Med. 337, 563–566.
Khachik R, Beecher GR, Goli MB, Lusby WR &, Smith Jr JC (1992): Separation and identification of carotenoids and their oxidation products in the extracts of human plasma. Anal. Chem. 64, 2111–2122.
Klipstein Grobusch K, Launer LJ, Geleijnse JM, Boeing H, Hofman A &, Witteman JC (2000): Serum carotenoids and atherosclerosis. The Rotterdam Study. Atherosclerosis 148, 49–56.
Kostic D, White WS &, Olson JA (1995): Intestinal absorption, serum clearance, and interactions between lutein and beta-carotene when administered to human adults in separate or combined oral doses. Am. J. Clin. Nutr. 62, 604–610.
Kromhout D (1989): Food consumption patterns in the Seven Countries Study. Seven Countries Study Research Group. Ann. Med. 21, 237–238.
LaRosa JC, Hunninghake D, Bush D, Criqui MH, Getz GS, Gotto AM, Grundy SM, Rakita L, Robertson RM, Weisfeldt ML et al (1990): The cholesterol facts. A summary of the evidence relating dietary fats, serum cholesterol, and coronary heart disease. A joint statement by the American Heart Association and the National Heart, Lung, and Blood Institute. The Task Force on Cholesterol Issues, American Heart Association. Circulation 81, 1721–1733.
Le Marchand L, Hankin JH, Kolonel LN, Beecher GR, Wilkens LR &, Zhao LP (1993): Intake of specific carotenoids and lung cancer risk. Cancer Epidemiol. Biomarkers Prev. 2, 183–187.
Lee A, Thurnham DI &, Chopra M (2000): Consumption of tomato products with olive oil but not sunflower oil increases the antioxidant activity of plasma. Free Rad. Biol. Med. 29, 1051–1055.
Lessin W, Catigani G &, Schwartz S (1997): Quantification of cis-trans isomers of provitamin A carotenoids in fresh fruits and vegetables. J. Agric. Food Chem. 45, 3728–3732.
Mensink RP &, Katan MB (1992): Effect of dietary fatty acids on serum lipids and lipoproteins. A meta-analysis of 27 trials. Arterioscler. Thromb. 12, 911–919.
Michaud DS, Feskanich D, Rimm EB, Colditz GA, Speizer FE, Willett WC &, Giovannucci E (2000): Intake of specific carotenoids and risk of lung cancer in 2 prospective US cohorts. Am. J. Clin. Nutr. 72, 990–997.
Micozzi MS, Brown ED, Edwards BK, Bieri JG, Taylor PR, Khachik F, Beecher GR &, Smith Jr JC (1992): Plasma carotenoid response to chronic intake of selected foods and beta-carotene supplements in men. Am. J. Clin. Nutr. 55, 1120–1125.
Miller NJ, Sampson J, Candeias LP, Bramley PM &, Rice-Evans CA (1996): Antioxidant activities of carotenes and xanthophylls. FEBS Lett. 384, 240–242.
Mortensen A, Skibsted LH, Sampson L, Rice Evans C &, Everett SA (1997): Comparative mechanisms and rates of free radical scavenging by carotenoid antioxidants. FEBS Lett. 418, 91–97.
Norrish AE, Jackson RT, Sharpe SJ &, Skeaff CM (2000): Prostate cancer and dietary carotenoids. Am. J. Epidemiol. 151, 119–123.
Olmedilla Alonso B, Granado Lorencio R, Gil Martinez E, Blanco Navarro I &, Rojas Hidalgo E (1997): Serum status of carotenoids in control subjects and its relation to the diet. Nutr. Hosp. 12, 245–249.
Olmedilla B, Granado F, Blanco I &, Rojas Hidalgo E (1994): Seasonal and sex-related variations in six serum carotenoids, retinol, and alpha-tocopherol. Am. J. Clin. Nutr. 60, 106–110.
Olmedilla B, Granado F, Southon S, Wright AJA, Blanco I, Gil-Martinez E, van den Berg H, Thurnham D, Corridan B, Chopra M &, Hininger I (2002): A European multicentre, placebo-controlled supplementation study with alpha-tocopherol, carotene-rich palm oil, lutein or lycopene: analysis of serum responses. Clin. Sci. 102, 447–456.
Omenn GS, Goodman GE, Thonquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Valanis B, Williams JH, Bamhart S &, Hammar S (1996): Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N. Engl. J. Med. 334, 1150–1155.
Ornish D, Scherwitz LW, Billings JH, Brown SE, Gould KL, Merritt TA, Sparler S, Annstrong WT, Ports TA, Kirkeeide RL, Hogeboom C &, Brand RJ (1998): Intensive lifestyle changes for reversal of coronary heart disease. JAMA 280, 2001–2007.
Paiva SA &, Russell RM (1999): Beta-carotene and other carotenoids as antioxidants. J. Am. Coll. Nutr. 18, 426–433.
Parks EJ &, Hellerstein MK (2000): Carbohydrate-induced hypertri-acylglycerolemia: historical perspective and review of biological mechanisms. Am. J. Clin. Nutr. 71, 412–433.
Porrini M, Riso P &, Testolin G (1998): Absorption of lycopene from single or daily portions of raw and processed tomato. Br. J. Nutr. 80, 353–361.
Prince MR &, Frisoli JK (1993): Beta-carotene accumulation in serum and skin. Am. J. Clin. Nutr. 57, 175–181.
Rissanen TH, Voutilainen S, Nyyssorien K, Lakka TA, Sivenius J, Salonen R, Kaplan GA &, Salonen JT (2001): Low serum lycopene concentration is associated with an excess incidence of acute coronary events and stroke: the Kuopio Ischaemic Heart Disease Risk Factor Study. Br. J. Nutr. 85, 749–754.
Rock CL, Swendseid ME, Jacob RA &, McKee RW (1992): Plasma carotenoid levels in human subjects fed a low carotenoid diet. J. Nutr. 122, 96–100.
Roodenburg AJ, Leenen R, van het Hof KH, Weststrate JA &, Tijburg LB (2000): Amount of fat in the diet affects bioavailability of lutein esters but not of alpha-carotene, beta-carotene, and vitamin E in humans. Am. J. Clin. Nutr. 71, 1187–1193.
Schierle L, Bretzel W, Buehler I, Faccin N, Hess D, Steiner K &, Schueep W (1997): Content and isomeric ratio of lycopene in food and human blood plasma. Food Chem. 59, 459–465.
Seddon JM, Ajani UA, Sperduto RD, Hiller R, Blair N, Burton TC, Farber MD, Gragoudas ES, Haller J, Miller DT et al. (1994): Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration Eye Disease Case-Control Study Group. JAMA 272, 1413–1420.
Stahl W &, Sies H (1992): Uptake of lycopene and its geometrical isomers is greater from heat-processed than from unprocessed tomato juice in humans. J. Nutr. 122, 2161–2166.
Street DA, Comstock GW, Salkeld RM, Schuep W &, Klag MJ (1994): Serum antioxidants and myocardial infarction. Are low levels of carotenoids and alpha-tocopherol risk factors for myocardial infarction? Circulation 90, 1154–1161.
Su Q, Rowley KG &, O'Dea K (1999): Stability of individual carotenoids, retinol and tocopherols in human plasma during exposure to light and after extraction. J. Chromatogr. B. Biomed. Sci. Appl. 729, 191–198.
USDA (1998): USDA-NCC Carotenoid Database for US Foods: National Cancer Institute. http://www.nal.usda.gov/fnic/foodcomp/Data/car98/car98.html.
Velthuis te Wierik EJ, van den Berg H, Weststrate JA, van het Hof KH &, de Graaf C (1996): Consumption of reduced-fat products: effects on parameters of antioxidative capacity. Eur. J. Clin. Nutr. 50, 214–219.
Watts GF, Lewis B, Brunt JN, Lewis ES, Coltart DJ, Smith LD, Mann JI &, Swan AV (1992): Effects on coronary artery disease of lipid-lowering diet, or diet plus cholestyramine, in the St Thomas'Atherosclerosis Regression Study (STARS). Lancet 339, 563–569.
Wei W, Kim Y &, Boudreau N (2001): Association of smoking with serum and dietary levels of antioxidants in adults: NHANES III, 1988-1994. Am. J. Public Health 91, 258–264.
WHO (1990): Diet, nutrition and the prevention of chronic diseases. Geneva: WHO.
Williams AW, Boileau TW &, Erdman Jr JW (1998): Factors influencing the uptake and absorption of carotenoids. Proc. Soc. Exp. Biol. Med. 218, 106–118.
Yeum KJ, Booth SL, Sadowski JA, Liu C, Tang G, Krinsky NI &, Russell RM (1996): Human plasma carotenoid response to the ingestion of controlled diets high in fruits and vegetables. Am. J. Clin. Nutr. 64, 594–602.
Zeegers MP, Goldbohm RA &, van den Brandt PA (2001): Are retinol, vitamin C, vitamin E, folate and carotenoids intake associated with bladder cancer risk? Results from the Netherlands Cohort Study. Br. J. Cancer. 85, 977–983.
Acknowledgements
Dr Su Qing for performing the biochemical analysis of carotenoids.
Author information
Authors and Affiliations
Contributions
Guarantor: M Ball
Contributors: KA was involved in the design, subject recruitment, conduct of the study, dietary and laboratory analysis, statistical analyses and writing of the paper. EA was involved in the day-to-day running and laboratory analysis of the study. MB was involved in the design of the study, supervision of the running of the study and writing of the paper.
Corresponding author
Rights and permissions
About this article
Cite this article
Ahuja, K., Ashton, E. & Ball, M. Effects of a high monounsaturated fat, tomato-rich diet on serum levels of lycopene. Eur J Clin Nutr 57, 832–841 (2003). https://doi.org/10.1038/sj.ejcn.1601617
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ejcn.1601617
Keywords
This article is cited by
-
Back-transformation of treatment differences—an approximate method
European Journal of Clinical Nutrition (2014)
-
Tomato-based food products for prostate cancer prevention: what have we learned?
Cancer and Metastasis Reviews (2010)