Abstract
Background: Hypercholesterolemia is an important risk factor for cardiovascular disease. Orally administered chitosan binds lipids in the small intestine and reduces their absorption. Chitosan has been shown to decrease serum cholesterol in animal and human studies. This study investigated the effectiveness of chitosan in reducing serum cholesterol without concomitant diet therapy.
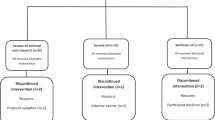
Methods: Ninety female volunteers (age 34–70 y) with confirmed mild to moderate hypercholesterolemia were enrolled into the study. They were randomly assigned to receive chitosan (1.2 g per day) or placebo in a double-blind manner. Serum lipids, body weight and adverse events were assessed at baseline and after 28 and 56 days of treatment. Subjects maintained their usual diet and documented the type and gross amount of food consumed.
Results: Eighty-four subjects (41 chitosan, 43 placebo) were included in the analysis. Chitosan significantly (F=3.19, P=0.04) reduced total cholesterol compared to placebo. In a subgroup of subjects with over 60 y of age, chitosan group significantly reduced total and LDL cholesterol (F=4.21, P=0.02, and F=3.46, P=0.04, respectively) compared with placebo. Adverse effects were few; no serious events were reported.
Conclusions: Our results demonstrate that chitosan is safe and effective for lowering cholesterol. However, the effect of chitosan for decreasing cholesterol is mild.
Sponsorship: Shimane Institute of Health Science, Izumo, Japan.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Arai, J, Tamama, K, Ishiyama, N, Kimura, T, Kuwabara, A, Kanda, T, Fukumura, Y & Kobayashi, I (2000). Contemplation of age-adjusted standard values of lipid profiles in Japan. J. Med., 31, 262–270.
Bucher, HC, Griffith, LE & Guyatt, GH (1998). Effect of HMGcoA reductase inhibitors on stroke. A meta-analysis of randomized, controlled trials. Ann. Intern. Med., 128, 89–95.
Colombo, P & Sciutto, AM (1996). Nutritional aspects of chitosan employment in hypocaloric diet. Acta Toxicol. Ther., 17, 278–302.
Davidson, MH, Maki, KC, Karp, SK & Ingram, KA (2002). Management of hypercholesterolaemia in postmenopausal women. Drugs Aging, 19, 169–178.
Ebihara, K & Schneeman, BO (1989). Interaction of bile acids, phospholipids, cholesterol and triglyceride with dietary fibers in the small intestine of rats. J. Nutr., 119, 1100–1106.
Gallaher, CM, Munion, J, Hesslink, R Jr, Wise, J & Gallaher, DD (2000). Cholesterol reduction by glucomannan and chitosan is mediated by changes in cholesterol absorption and bile acid and fat excretion in rats. J. Nutr., 130, 2753–2759.
Giustina, A & Ventura, P (1995). Weight-reducing regimens in obese subjects: effects of a new dietary fibre integrator. Acta Toxicol. Ther., 16, 199–214.
Kannel, WB (1995). Range of serum cholesterol values in the population developing coronary artery disease. Am. J. Cardiol., 76, 69C–77C.
Lee, AJ & Maddix, DS (2001). Rhabdomyolysis secondary to a drug interaction between simvastatin and clarithromycin. Ann. Pharmacother., 35, 26–31.
Martin, MJ, Hulley, SB, Browner, WS, Kuller, LH & Wentworth, D (1986). Serum cholesterol, blood pressure, and mortality: implications from a cohort of 361,662 men. Lancet, 2, 933–936.
Moghadasian, MH, Mancini, GB & Frohlich, JJ (2000). Pharmacotherapy of hypercholesterolaemia: statins in clinical practice. Expert. Opin. Pharmacother., 1, 683–695.
Muzzarelli, RA, Ilari, P & Petrarulo, M (1994). Solubility and structure of N-carboxymethylchitosan. Int. J. Biol. Macromol., 16, 177–180.
Pekkanen, J, Linn, S, Heiss, G, Suchindran, CM, Leon, A, Rifkind, BM & Tyroler, HA (1990). Ten-year mortality from cardiovascular disease in relation to cholesterol level among men with and without preexisting cardiovascular disease. New Engl. J. Med., 322, 1700–1707.
Pittler, MH, Abbot, NC, Harkness, EF & Ernst, E (1999). Randomized, double-blind trial of chitosan for body weight reduction. Eur. J. Clin. Nutr., 53, 379–381.
Rackley, CE (2002). Strategies for the prevention of atherosclerotic progression in women. Cardiol. Rev., 10, 119–125.
Rose, G, Hamilton, PS, Keen, H, Reid, DD, McCartney, P & Jarrett, RJ (1977). Myocardial ischaemia, risk factors and death from coronary heart disease. Lancet, 1, 105–109.
Sacks, FM, Pfeffer, MA, Moye, LA, Rouleau, JL, Rutherford, JD, Cole, TG, Brown, L, Warnica, JW, Arnold, JM, Wun, CC, Davis, BR & Braunwald, B (1996). The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. New Engl. J. Med., 335, 1001–1009.
Sharrett, AR, Ballantyne, CM, Coady, SA, Heiss, G, Sorlie, PD, Catellier, D & Patsch, W (2001). Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins a-i and b, and hdl density subfractions: the atherosclerosis risk in communities (aric) study. Circulation, 104, 1108–1113.
Shepherd, J, Cobbe, SM, Ford, I, Isles, CG, Lorimer, AR, MacFarlane, PW, McKillop, JH & Packard, CJ (1995). Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. New Engl. J. Med., 333, 1301–1307.
Sheu, WH, Jeng, CY, Young, MS, Le, WJ & Chen, YT (2000). Coronary artery disease risk predicted by insulin resistance, plasma lipids, and hypertension in people without diabetes. Am. J. Med. Sci., 319, 84–88.
Sugano, M, Fujikawa, T, Hiratsuji, Y, Nakashima, K, Fukuda, N & Hasegawa, Y (1980). A novel use of chitosan as a hypocholesterolemic agent in rats. Am. J. Clin. Nutr., 33, 787–793.
Veneroni, G, Veneroni, F, Contos, S, Tripodi, S, de Bernardi, M, Guarino, C & Marleti, AM (1996). Effect of a new chitosan dietary integrator and hypocaloric diet on hyperlipidemia and overweight in obese patients. Acta Toxicol. Ther., 17, 53–70.
Wilson, PW & Kannel, WB (1993). Hypercholesterolemia and Coronary Risk in the Elderly: the Framingham Study. Am. J. Geriatr. Cardiol., 2, 56
Wuolijoki, E, Hirvela, T & Ylitalo, P (1999). Decrease in serum LDL cholesterol with microcrystalline chitosan. Meth. Find. Exp. Clin. Pharmac., 21, 357–361.
Yihua, YU & Binglin, HE (1997). A new low density lipoprotein (LDL) adsorbent. Artif. Cells Blood Substit. Immobil. Biotechnol., 25, 445–450.
Acknowledgements
The authors thank Kazuo Matsumoto, Access Laboratory for statistical data analysis, Sadaki Kikkawa, More Fresh Co. Ltd, and Chizuko Yamane for assistance with setting up the trial. This work was supported by Medical Research Grants from the Shimane Institute of Health Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bokura, H., Kobayashi, S. Chitosan decreases total cholesterol in women: a randomized, double-blind, placebo-controlled trial. Eur J Clin Nutr 57, 721–725 (2003). https://doi.org/10.1038/sj.ejcn.1601603
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ejcn.1601603
Keywords
This article is cited by
-
A review on the immunomodulatory properties of functional nutraceuticals as dietary interventions for children to combat COVID-19 related infections
Food Production, Processing and Nutrition (2023)
-
Management of metabolic syndrome by nutraceuticals prepared from chitosan and ferulic acid with or without beta-sitosterol and their nanoforms
Scientific Reports (2023)
-
Chitosan oligosaccharide (GO2KA1) improves postprandial glycemic response in subjects with impaired glucose tolerance and impaired fasting glucose and in healthy subjects: a crossover, randomized controlled trial
Nutrition & Diabetes (2019)
-
A Review of the Efficacy, Safety, and Clinical Implications of Naturally Derived Dietary Supplements for Dyslipidemia
American Journal of Cardiovascular Drugs (2017)
-
Characteristics and consumer acceptance of healthier meat and meat product formulations—a review
Journal of Food Science and Technology (2012)