Abstract
Objectives: To measure the availability of oxalate normally extracted when making tea from two commercially available black teas bought from a supermarket in Christchurch, New Zealand in July 2001.
Design, subjects and intervention: A randomized double crossover study. Six students and four staff consumed six cups of each brand of tea both with and without added milk over a 24 h period. A total urine collection was taken for the initial 6 h followed by a further 18 h. The oxalate content of the urine voided was measured using an enzyme kit method and the availability of the soluble oxalate consumed was measured for the 6 h and the total 24 h sample.
Setting: University campus.
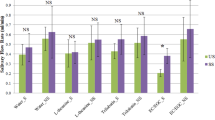
Results: The mean soluble oxalate content of black tea in the two different commercial tea bags was respectively 6.1 and 6.3 mg soluble oxalate/g tea. The mean availability of the oxalate extracted from tea measured over a 6 h period ranged from 1.9 to 4.7% when tea was consumed without milk. The availability of the soluble oxalate from tea ranged from −3.0 to 2.3% for each of the two brands of tea investigated over a 24 h period.
Conclusion: These studies show that consuming black tea on a daily basis will lead to a moderate intake of soluble oxalate each day, however the consumption of tea with milk on a regular basis will result in the absorption of very little oxalate from tea.
Sponsorship: Lincoln University.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Albihn, PBE & Savage, GP (2001a). The effect of cooking on the location and concentration of oxalate in three cultivars of New Zealand-grown oca (Oxalis tuberosa Mol). J. Sci. Food Agric., 81, 1–7.
Albihn, PBE & Savage, GP (2001b). The bioavailability of oxalate from oca (Oxalis tuberosa). J. Urol., 166, 420–422.
Barilla, DE, Notz, C, Kennedy, BS & Pak, CYC (1978). Renal oxalate excretion following oral loads in patients with ileal disease and with renal and absorptive hypercalciurias, effect of calcium and magnesium. Am. J. Med., 64, 579–585.
Brinkley, L, MgGuire, J, Gregory, J & Pack, CYC (1981). Bioavailability of oxalate in foods. Urology, 17, 534–553.
Brinkley, LJ, Gregory, J & Pak, CYC (1990). A further study of oxalate bioavailability. J. Urol., 144, 94–99.
Charrier, MJS, Savage, GP & Vanhanen, L (2002). Oxalate content and calcium binding capacity of tea and herbal teas. Asia Pacific J. Clin. Nutr., (in press)
Curhan, GC, Willett, WC & Rimm, EB et al (1996). Prospective study of beverage use and the risk of kidney stones. Am. J. Epidemiol., 143, 240–247.
Curhan, GC, Willett, WC, Speizer, FE & Stampfer, MJ (1998). Beverage use and risk for kidney stones in women. Ann. Intern. Med., 128, 534–540.
Finch, AM, Kasidas, GP & Rose, GA (1981). Urine composition in normal subjects after oral ingestion of oxalate-rich foods. Clin. Sci., 60, 411–418.
Heaney, RP, Weaver, CM & Recker, RR (1988). Calcium absorbability from spinach. Am. J. Clin. Nutr., 47, 707–709.
Holmes, RP, Goodman, HO & Assimos, DG (1996). Metabolic effects of an oxalate-free-diet. In:Urolithias, ed. CYC Pak, M I Resnick & GM Preminger, pp.167–168, Dallas, TX: Millet
Liebman, M & Chai, W (1997). Effect of dietary calcium on urinary oxalate excretion after oxalate loads. Am. J. Clin. Nutr., 65, 1453–1459.
Marangella, M, Futtero, M, Bruno, M & Linari, F (1982). Hyperoxaluria in ideopathic calcium stone disease: further evidence of intestinal hyperabsorbtion of oxalate. Clin. Sci., 63, 381–385.
Marshall, RW, Cochran, M & Hodgkinson, A (1972). Relationships between calcium and oxalic acid intake in the diet and their excretion in the urine of normal and renal-stone-forming subjects. Clin. Sci., 43, 91–99.
Masai, M, Ito, H & Kotake, T (1995). Effect of dietary intake on urinary oxalate excretion in calcium renal stone disease. Br. J. Urol., 76, 692–699.
Massey, LK (2000). Tea oxalate. Nutr. Rev., 58, 88–89.
Massey, LK, Roman-Smith, H & Sutton, RAL (1993). Effect of dietary oxalate and calcium on urinary oxalate and risk of formation of calcium oxalate kidney stones. J. Am. Diet. Assoc., 93, 901–906.
McKay, DW, Sevior, JP, Comerford, A, Vasdev, S & Massey, LK (1995). Herbal tea: An alternative to regular tea for those who form calcium oxalate stones. J. Am. Diet. Assoc., 95, 360–361.
Noonan, SC & Savage, GP (1999). Oxalate content of foods and its effect on humans. Asia Pacific J. Clin. Nutr., 8, 64–67.
Savage, GP, Vanhanen, L, Mason, SL & Ross, AB (2000). Effect of cooking on the soluble and insoluble oxalate content of some New Zealand foods. J. Food Comp. Anal., 13, 201–206.
Weaver, CM, Heaney, RP, Nickel, KP & Packard, PI (1997). Calcium bioavailability from high oxalate vegetables: Chinese vegetables, sweet potatoes, potatoes and rhubarb. J. Food. Sci., 62, 524–552.
Zarembski, M & Hodgkinson, A (1962). The oxalic content of English diets. Br. J. Nutr., 16, 627–663.
Acknowledgements
The authors wish to acknowledge Trevor Walmsley, Canterbury Health Laboratories, Christchurch, NZ, for his assistance with the analysis of urinary oxalate and Professor D McNeil, Plant Sciences, Lincoln University for his assistance with the statistical analysis of the data.
Author information
Authors and Affiliations
Contributions
Guarantor: GP Savage.
Contributors: MJSC carried out the study, LV provided technical support, GPS supervised the study and wrote the manuscript.
Corresponding author
Rights and permissions
About this article
Cite this article
Savage, G., Charrier, M. & Vanhanen, L. Bioavailability of soluble oxalate from tea and the effect of consuming milk with the tea. Eur J Clin Nutr 57, 415–419 (2003). https://doi.org/10.1038/sj.ejcn.1601572
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ejcn.1601572
Keywords
This article is cited by
-
Tea and coffee consumption and pathophysiology related to kidney stone formation: a systematic review
World Journal of Urology (2021)
-
Dietary recommendations and treatment of patients with recurrent idiopathic calcium stone disease
Urolithiasis (2016)
-
Nutrition in calcium nephrolithiasis
Journal of Translational Medicine (2013)
-
Effect of different brewing times on soluble oxalate content of loose-packed black teas and tea bags
Urolithiasis (2013)
-
Oxalate content of green tea of different origin, quality, preparation and time of harvest
Urological Research (2010)