Abstract
Objective: To give an overview of the association between tea consumption and iron status.
Methods: A PUBMED search was performed (up to June 2001) for all publications containing the words: tea and ferritin, h(a)emoglobin, iron status or an(a)emia. Sixteen studies were evaluated in groups with high (infants, children and premenopausal women) or low prevalence of iron deficiency (men and the elderly).
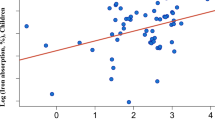
Results and Discussion: Of the 16 studies reviewed, six included infants and children, six premenopausal women, two men and two the elderly. In study groups with high prevalence of iron deficiency, tea consumption was inversely associated with serum ferritin and/or haemoglobin. The association disappeared when adjusting for confounding (dietary) factors, except for one study including 40% of iron deficient women. In groups with low prevalence of iron deficiency, tea consumption was not inversely associated with serum ferritin and/or haemoglobin. In those at risk for iron overload, such as middle-aged men, tea consumption may lower serum ferritin concentrations as reported in one study. This finding awaits further confirmation.
Conclusion: This overview shows that tea consumption does not influence iron status in Western populations in which most people have adequate iron stores as determined by serum ferritin concentrations. Only in populations of individuals with marginal iron status does there seem to be a negative association between tea consumption and iron status.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Beaton G . 1974 Epidemiology of iron deficiency In Iron in Biochemistry and Medicine, ed. A Jacobs & M Worwood 477–528 London: Academic Press
Bender D, Bender A . 1997 Iron In Nutrition: A Reference Handbook 394–406 New York: Oxford University Press
Brune M, Rossander L, Hallberg L . 1989 Iron absorption and phenolic compounds: importance of different phenolic structures Eur. J. Clin. Nutr. 43: 547–557
Cook J . 1990 Adaptation in iron metabolism Am. J. Clin. Nutr. 5l: 301–308
Cowin I, Emmond P, Emmett P, and the ALSPAC Study Team . 2001 Association between composition of the diet and haemoglobin and ferritin levels in 18-month-old children Eur. J. Clin. Nutr. 55: 278–286
Disler P, Lynch S, Charlton R, Torrance J, Bothwell T, Walker R, Mayet F . 1975 The effect of tea on iron absorption Gut 16: 193–200
Doyle W, Crawley H, Robert H, Bates C . 1999 Iron deficiency in older people: interactions between food and nutrient intakes with biochemical measures of iron; further analysis of the National Diet and Nutrition Survey of people aged 65 y and over Eur. J. Clin. Nutr. 53: 552–559
Fleming DJ, Jacques PF, Tucker KL, Massaro JM, D'Agostino RBS, Wilson PW, Wood RJ . 2001 Iron status of the free-living, elderly Framingham Heart Study cohort: an iron-replete population with a high prevalence of elevated iron stores Am. J. Clin. Nutr. 73: 638–646
Gabay C, Kushner I . 1999 Acute-phase proteins and other systemic responses to inflammation New Engl. J. Med. 340: 448–454
Galan P, Hercberg S, Soustre Y, Dop M, Dupin H . 1985 Factors affecting iron stores in French female students Hum. Nutr. Clin. Nutr. 39C: 279–287
Gibson S . 1999 Iron intake and iron status of preschool children: associations with breakfast cereals, vitamin C and meat Public Health Nutr. 2: 521–528
Hallberg L, Hulthen L . 2000 Prediction of dietary iron absorption: an algorithm for calculating absorption and bioavailability of dietary iron Am. J. Clin. Nutr. 71: 1147–1160
Hunt J, Roughead Z . 2000 Adaptation of iron absorption in men consuming diets with high or low iron bioavailability Am. J. Clin. Nutr. 71: 94–102
Imai K, Nakachi K . 1995 Cross sectional study of effects of drinking green tea on cardiovascular and liver diseases Br. Med. J. 310: 693–696
Kuvibidila S, Mbele V, Yu L, Ode D, Warrier R . 1992 The influence of tea consumption on iron status and anthropometry in young Zairean children Clin. Res. 40: 631A–
Looker AC, Dallman PR, Carroll MD, Gunter EW, Johnson CL . 1997 Prevalence of iron deficiency in the United States JAMA 277: 973–976
MacPhail P . 1998 Iron In Essentials of Human Nutrition ed. J Mann & A Truswell (eds) New York: Oxford University Press 137–149
Mehta S, Pritchard M, Stegman C . 1992 Contribution of coffee and tea to anemia among NHANES II participants Nutr. Res. 12: 209–222
Merhav H, Amitai Y, Palti H, Godfrey S . 1985 Tea drinking and microcytic anemia in infants Am. J. Clin. Nutr. 41: 1210–1213
Milman N, Ovesen L, Byg K, Graudal N . 1999 Iron status in Danes updated 1994. I: prevalence of iron deficiency and iron overload in 1332 men aged 40–70 y. Influence of blood donation, alcohol intake, and iron supplementation Ann. Hematol. 78: 393–400
Murray C, Lopez A . 1996 In Global Health Statistics: a Compendium of Incidence, Prevalence and Mortality Estimated for Over 2000 Conditions Geneva: WHO
Pate RR, Miller BJ, Davis JM, Slentz CA, Klingshirn LA . 1993 Iron status of female runners Int. J. Sport Nutr. 3: 222–231
Razagui I, Barlow P, Izmeth M, Taylor K . 1991 Iron status in a group of long-stay mentally handicapped menstruating women: some dietary considerations Eur. J. Clin. Nutr. 45: 331–340
Reddy M, Hurrell R, Cook J . 2000 Estimation of nonheme-iron bioavailability from meal composition Am. J. Clin. Nutr. 71: 937–943
Roebothan B, Chandra R . 1996 The contribution of dietary iron to iron status in a group of eldery subjects Int. J. Vit. Nutr. Res. 66: 66–70
Root M, Stephenson L, Parker R, Campbell T . 1999 Iron status of middle-aged women in five counties of rural China Eur. J. Clin. Nutr. 53: 199–206
Soustre Y, Dop M, Galan P, Hercberg S . 1986 Dietary determinants of the iron status in menstruating women Int. J. Vit. Nutr. Res. 56: 281–286
Thane CW, Walmsley CM, Bates CJ, Prentice A, Cole TJ . 2000 Risk factors for poor iron status in British toddlers: further analysis of data from the National Diet and Nutrition Survey of children aged 1.5–4.5 y Public Health Nutr. 3: 433–440
Van de V, ijver LPL, Kardinaal AFM, Charzewska J, Rotily M, Charles P, Maggiolini M, Ando S, Vaananen K . 1999 Calcium intake is weakly but consistently negatively associated with iron status in girls and women in six European countries J. Nutr. 129: 963–968
Wilson C, Grant C, Wall C . 1999 Iron deficiency anaemia and adverse dietary habits in hospitalised children N.Z. Med. J. 112: 203–206
Yen C-L, Su L . 1999 Tea consumption is inversely associated with iron status in serum Am. J. Epidemiol. 149: 259
Acknowledgements
The authors thank Dr Jianjun Zhang, Dr Evert Schouten and Dr Hugo Kesteloot for critically evaluating the manuscript. This study was supported by a grant from the Unilever Chair in Nutritional Epidemiology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Temme, E., Van Hoydonck, P. Tea consumption and iron status. Eur J Clin Nutr 56, 379–386 (2002). https://doi.org/10.1038/sj.ejcn.1601309
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ejcn.1601309
Keywords
This article is cited by
-
Thé, les interactions nutritionnelles avec le fer
Phytothérapie (2013)
-
The prevalence of nutritional anemia in pregnancy in an east Anatolian province, Turkey
BMC Public Health (2010)
-
Protective effects of crude garlic by reducing iron-mediated oxidative stress, proliferation and autophagy in rats
Journal of Molecular Histology (2010)
-
Sociodemographic correlates of food habits among school adolescents (12–15 year) in north Gaza Strip
BMC Public Health (2009)
-
Black tea – helpful or harmful? A review of the evidence
European Journal of Clinical Nutrition (2007)