Abstract
Objective: The objective of this study was to compare the effects of dietary monounsaturated fatty acids (MUFA), n-6 and n-3 polyunsaturated fatty acids (PUFA) on LDL composition and oxidizability.
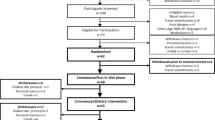
Design, setting and subjects: Sixty-nine healthy young volunteers, students at a nearby college, were included. Six subjects withdrew because of intercurrent illness and five withdrew because they were unable to comply with the dietary regimen.
Interventions: The participants received a 2-week wash-in diet rich in saturated fatty acids (SFA) followed by diets rich in refined olive oil, rapeseed oil or sunflower oil for 4 weeks. Intakes of vitamin E and other antioxidants did not differ significantly between the diets.
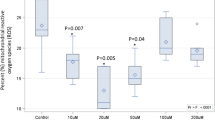
Results: At the end of the study, LDL oxidizability was lowest in the olive oil group (lag time: 72.6 min), intermediate in the rapeseed oil group (68.2 min) and highest in the sunflower oil group (60.4 min, P<0.05 for comparison of all three groups). Despite wide variations in SFA intake, the SFA content of LDL was not statistically different between the four diets (25.8–28.5% of LDL fatty acids). By contrast, the PUFA (43.5%–60.5% of LDL fatty acids) and MUFA content of LDL (13.7–29.1% of LDL fatty acids) showed a wider variability dependent on diet.
Conclusions: Enrichment of LDL with MUFA reduces LDL susceptibility to oxidation. As seen on the rapeseed oil diet this effect is independent of a displacement of higher unsaturated fatty acids from LDL. Evidence from this diet also suggests that highly unsaturated n-3 fatty acids in moderate amounts do not increase LDL oxidizability when provided in the context of a diet rich in MUFA.
Sponsorship: This work was supported by the Central Marketing Agency of the German Agricultural Industry (CMA), the German Union for the Promotion of Oil- and Protein Plants (UFOP), the Austrian Science Foundation, project F00709 (to P.M.A.) and the Brökelmann Ölmühle Company, Hamm, Germany.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Abbey M, Belling GB, Noakes M, Hirata F, Nestel PJ . 1993 Oxidation of low-density lipoproteins: intraindividual variability and the effect of dietary linoleate supplementation Am. J. Clin. Nutr. 57: 391–398
Berry EM, Eisenberg S, Haratz D, Friedlander Y, Norman Y, Kaufmann NA, Stein Y . 1991 Effects of diets rich in monounsaturated fatty acids on plasma lipoproteins—the Jerusalem Nutrition Study: high MUFAs vs high PUFAs Am. J. Clin. Nutr. 53: 899–907
Bonanome A, Pagnan A, Biffanti S, Opportuno A, Sorgato F, Dorella M, Maiorino M, Ursini F . 1992 Effect of dietary monounsaturated and polyunsaturated fatty acids on the susceptibility of plasma low density lipoproteins to oxidative modification Arterioscler. Thromb. 12: 529–533
Brude IR, Drevon CA, Hjermann I, Seljeflot I, Lund-Katz S, Saarem K, Sandstad B, Solvoll K, Halvorsen B, Arnesen H, Nenseter MS . 1997 Peroxidation of LDL from combined hyperlipidemic male smokers supplied with omega-3 fatty acids and antioxidants Arterioscler. Thromb. Vasc. Bio. 17: 2576–2588
Burstein M, Samaille J . 1960 Sur un dosage rapide du cholesterol lié aux alpha- et aux betalipoproteins du sérum Clin. Chim. Acta 5: 609–614
Chung BH, Wilkinson T, Geer JC, Segrest JP . 1980 Preparative and quantitative isolation of plasma lipoproteins: rapid, single discontinuous density gradient ultracentrifugation in a vertical rotor J. Lipid Res. 21: 284–291
de Lorgeril M, Renaud S, Mamelle N, Salen P, Martin JL, Monjaud I, Guidollet J, Touboul P, Delaye J . 1994 Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease Lancet 343: 8911: 1454–1459 [Published erratum appears in Lancet 1995 Mar 18; 345, (8951): 738.]
Esterbauer H, Striegl G, Puhl H, Rotheneder M . 1989 Continuous monitoring of in vitro oxidation of human low density lipoprotein Free Radic. Res. Commun. 6: 67–75
Esterbauer H, Gebicki J, Puhl H, Jurgens G . 1992 The role of lipid peroxidation and antioxidants in oxidative modification of LDL Free Radic. Biol. Med. 13: 341–390
Gardner CD, Kraemer HC . 1995 Monounsaturated versus polyunsaturated dietary fat and serum lipids. A meta-analysis Arterioscler. Thromb. Vasc. Biol. 15: 1917–1927
Grundy SM . 1997 What is the desirable ratio of saturated, polyunsaturated, and monounsaturated fatty acids in the diet? Am. J. Clin. Nutr. 66: 4 Suppl: 988S–990S
Higdon JV, Liu J, Du SH, Morrow JD, Ames BN, Wander RC . 2000 Supplementation of postmenopausal women with fish oil rich in eicosapentaenoic acid and docosahexaenoic acid is not associated with greater in vivo lipid peroxidation compared with oils rich in oleate and linoleate as assessed by plasma malondialdehyde and F(2)-isoprostanes. (In Process Citation) Am. J. Clin. Nutr. 72: 714–722
Hu FB, Stampfer MJ, Manson JE, Rimm E, Colditz GA, Rosner BA, Hennekens CH, Willett WC . 1997 Dietary fat intake and the risk of coronary heart disease in women New Engl. J. Med. 337: 1491–1499
Jialal I, Fuller CJ, Huet BA . 1995 The effect of alpha-tocopherol supplementation on LDL oxidation. A dose–response study Arterioscler. Thromb. Vasc. Biol. 15: 190–198
Keys A . Seven Countries: a Multivariate Analysis of Health and Coronary Heart Disease. Harvard University Press: Harvard, MA 1980
Kris-Etherton PM . 1999 AHA Science Advisory. Monounsaturated fatty acids and risk of cardiovascular disease. American Heart Association. Nutrition Committee Circulation 100: 1253–1258
Lepage G, Roy CL . 1999 Direct transesterification of all classes of lipids in a one-step reaction J. Lipid Res. 27: 114–120
Ma J, Folsom AR, Lewis L, Eckfeldt JH . 1997 Relation of plasma phospholipid and cholesterol ester fatty acid composition to carotid artery intima-media thickness: the Atherosclerosis Risk in Communities (ARIC) Study Am. J. Clin. Nutr. 65: 551–559
Mata P, Alonso R, Lopez-Farre A, Ordovas JM, Lahoz C, Garces C, Caramelo C, Codoceo R, Blazquez E, de Oya M . 1996 Effect of dietary fat saturation on LDL oxidation and monocyte adhesion to human endothelial cells in vitro Arterioscler. Thromb. Vasc. Biol. 16: 1347–1355
McGrath LT, Brennan GM, Donnelly JP, Johnston GD, Hayes JR, McVeigh GE . 1996 Effect of dietary fish oil supplementation on peroxidation of serum lipids in patients with non-insulin dependent diabetes mellitus Atherosclerosis 121: 275–283
Mensink RP, Katan MB . 1992 Effect of dietary fatty acids on serum lipids and lipoproteins. A meta-analysis of 27 trials Arterioscler. Thromb. 12: 911–919
Mironova M, Virella G, Lopez-Virella MF . 1996 Isolation and characterization of human antioxidized LDL autoantibodies Arterioscler. Thromb. 16: 222–229
Mori TA, Dunstan DW, Burke V, Croft KD, Rivera JH, Beilin LJ, Puddey IB . 1999 Effect of dietary fish and exercise training on urinary F2-isoprostane excretion in non-insulin-dependent diabetic patients Metabolism 48: 1402–1408
Mori TA, Puddey IB, Burke V, Croft KD, Dunstan DW, Rivera JH, Beilin LJ . 2000 Effect of omega3 fatty acids on oxidative stress in humans. GC-MS measurement of urinary F2-isoprostane excretion. [In Process Citation.] Redox. Rep. 51: 45–46
Nestel PJ, Pomeroy SE, Sasahara T, Yamashita T, Liang YL, Dart AM, Jennings GL, Abbey M, Cameron JD . 1997 Arterial compliance in obese subjects is improved with dietary plant n-3 fatty acid from flaxseed oil despite increased LDL oxidizability Arterioscler. Thromb. Vasc. Biol. 17: 1163–1170
Palinski W, Rosenfeld ME, Ylä-Herttuala S, Gurtner GC, Socher SS, Butler SW, Parthasarathy S, Carew T, Steinberg D, Witztum JL . 1989 Low density lipoprotein undergoes oxidative modification in vivo Proc. Natl. Acad. Sci. USA 86: 1372–1376
Princen HMG, van-Duyvenvoorde W, Buytenhek R, van-der-Laarse A, van-Poppel G, Gevers Leuven JA, van-Hinsbergh VWM . 1995 Supplementation with low doses of vitamin E protects LDL from lipid peroxidation in men and women Arterioscler. Thromb. Vasc. Biol. 15: 325–333
Reaven P . 1996 The role of dietary fat in LDL oxidation and atherosclerosis Nutr. Metab. Cardiovasc. Dis. 6: 57–64
Reaven P, Parthasarathy S, Grasse BJ, Miller E, Almazan F, Mattson FH, Khoo JC, Steinberg D, Witztum JL . 1991 Feasibility of using on oleate-rich diet to reduce the susceptibility of low-density lipoprotein to oxidative modification in humans Am. J. Clin. Nutr. 54: 701–706
Reaven P, Parthasarathy S, Grasse BJ, Miller E, Steinberg D, Witztum JL . 1993 Effects of oleate rich and linoleate rich diets on the susceptibility of low density lipoprotein to oxidative modification in mildly hypercholesterolemic subjects J. Clin. Invest. 91: 668–676
Reaven PD, Grasse BJ, Tribble DL . 1994 Effects of linoleate-enriched and oleate-enriched diets in combination with alpha-tocopherol on the susceptibility of LDL and LDL subfractions to oxidative modification in humans Arterioscler. Thromb. 14: 557–566
Regnstrom J, Nilsson J, Tornvall P, Landou C, Hamsten A . 1992 Susceptibility to low-density lipoprotein oxidation and coronary atherosclerosis in man Lancet 339: 8803: 1183–1186
Röschlau P, Bernt E, Gruber Z . 1974 Enzymatische Bestimmung des Gesamtcholesterins im Serum Z. Klin. Chem. Klin. Biochem. 12: 403–408
Sorensen NS, Marckmann P, Hoy CE, van-Duyvenvoorde W, Princen HM . 1998 Effect of fish-oil-enriched margarine on plasma lipids, low-density-lipoprotein particle composition, size, and susceptibility to oxidation Am. J. Clin. Nutr. 68: 235–241
Turpeinen AM, Alfthan G, Valsta L, Hietanen E, Salonen JT, Schunk H, Nyyssonen K, Mutanen M . 1995 Plasma and lipoprotein lipid-peroxidation in humans on sunflower and rapeseed oil diets Lipids 30: 485–492
Westhuyzen J . 1997 The oxidation hypothesis of atherosclerosis: an update Ann. Clin. Lab. Sci. 27: 1–10
World Health Organization . The World Health Report 1997. Conquering suffering, enriching humanity. Geneva: World Health Organization 1997
Ylä-Herttuala S, Palinski W, Rosenfeld ME, Parthasarathy S, Carew T, Butler S, Witztum JL, Steinberg D . 1989 Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man J. Clin. Invest. 84: 1086–1095
Acknowledgements
We are indebted to B Pieke, B Berning, J Harmsen and particularly W Hanekamp and E Gramenz for excellent technical assistance; to R Schmidt, Dr R Junker and Dr G Bannenberg for performing the venipunctures; to Dr Arnold von Eckardstein for valuable discussion; to M Nestola and J Ackermann at the Bildungszentrum der Bundesfinanzverwaltung for their generous cooperation; to E Ostermann and the Camphill Werkstätten, Steinfurt, for supplying the oil-enriched bread and cake; to M Stennecken and Dr H Schulte for statistical analyses; to the Homann Company, Dissen, Germany, and particularly W Heimhalt for supplying the specially manufactured margarine; and last but not least to the study subjects for participation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kratz, M., Cullen, P., Kannenberg, F. et al. Effects of dietary fatty acids on the composition and oxidizability of low-density lipoprotein. Eur J Clin Nutr 56, 72–81 (2002). https://doi.org/10.1038/sj.ejcn.1601288
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ejcn.1601288
Keywords
This article is cited by
-
High fat diet and PCSK9 knockout modulates lipid profile of the liver and changes the expression of lipid homeostasis related genes
Nutrition & Metabolism (2023)
-
Modulation of endothelium function by fatty acids
Molecular and Cellular Biochemistry (2022)
-
Fatty acid desaturase-2 (ahFAD2) mutant alleles in peanut (Arachis hypogaea L.) pre-breeding lines: an insight into the source, features, discourse, and selection of novel pre-breeding lines
Genetic Resources and Crop Evolution (2021)
-
Comparing the simultaneous determination of cis- and trans-palmitoleic acid in fish oil using HPLC and GC
Lipids in Health and Disease (2019)
-
Improving oil quality by altering levels of fatty acids through marker-assisted selection of ahfad2 alleles in peanut (Arachis hypogaea L.)
Euphytica (2018)