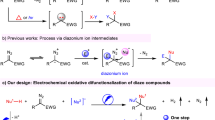
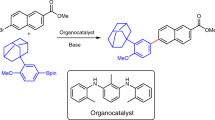
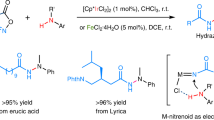
Abstract
THE formation of diazonium complexes from unsaturated amines is not a unique property of the aromatic series. Many, but not all, unsaturated heterocyclic amines respond similarly1. With aliphatic amino-compounds, nitrous acid, under certain conditions, may give a diazo-grouping but not an ionizable diazo-derivative. In the literature it would appear that aminoguanidine is the only straightchain compound so far recorded as being in line with the aromatic amines. Even in this example the tendency is to form an intermediate heterocyclic compound, and it is only under special conditions that a straight-chain diazonium derivative is obtained.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Morgan and Reilly, J. Chem. Soc., 103, 808, 1494 (1913); 105, 436 (1914); 109, 155 (1916). Reilly and Madden, J. Chem. Soc., 127, 2936 (1925); 134, 815 (1929). Reilly and MacSweeney, Proc. Roy. Irish Acad., 39, B, 497 (1930).
Thiele, Ann., 270, 46 (1892).
Hantzsch and Vagt, Ann., 339, 314 (1900).
Hofmann and Hock, Ber. 43, 1866 (1910).
Hofmann and Hock, Ber., 43, 682 (1910).
Norris-Shreve, Carter and Willis, Ind. Eng. Chem., 36, 472 (1936).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
REILLY, J., TEEGAN, J. & CAREY, M. Coupling of Diazonium Complexes from an Aliphatic Base. Nature 159, 643–644 (1947). https://doi.org/10.1038/159643a0
Issue Date:
DOI: https://doi.org/10.1038/159643a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.