Abstract
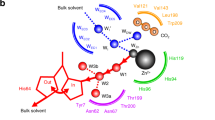
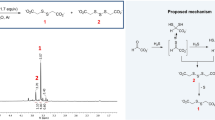
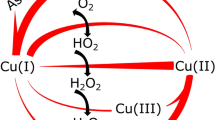
IN a recent letter in NATURE1, Keilin and Mann question the zinc content of carbonic anhydrase reported by us. The facts seem to be that they have obtained an enzyme preparation (which they believe is highly purified) having a zinc content of 0·3–0·33 per cent2, and we have obtained a preparation (which we likewise feel we have shown to be highly purified) having a zinc content of 0·2–0·23 per cent3. Realizing that criticism can be directed against the determination of any low zinc content, whether the method used be the dithizone method employed by Keilin and Mann or the method of Sahyun and Feldkamp used by us, we conducted preliminary experiments and reported4 that "Before zinc estimations were conducted on the enzyme, analyses were made on a sample of zinc-insulin crystals with the same technique as was used in estimating the zinc in the enzyme. Duplicate results agree within 4 per cent. Moreover, these results were in good agreement with the metal content of the crystals as calculated from ash determinations. Along with each estimation of the zinc content of an unknown sample it was routine procedure to determine likewise the zinc content of a standard zinc solution". In all our estimations of the zinc content of the enzyme preparations, care was taken that a reasonable quantity of material was used. Recently Prof. Thode of McMaster University made polarographic determinations of the zinc content of our enzyme preparation and found it to be 0.22 per cent. We feel that the comparatively low zinc values reported by us are not attributable to the method of determination used in our work.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Keilin, D., and Mann, T., NATURE, 153, 107 (1944).
Keilin, D., and Mann, T., Biochem. J., 34, 1163 (1940).
Scott, D. A., and Fisher, A. M., J. Biol. Chem., 144, 371 (1942).
Scott, D. A., and Mendive, J. R., J. Biol. Chem., 140, 445 (1941).
Scott, D. A., and Mendive, J. R., J. Biol. Chem., 139, 661 (1941).
Meldram, N. U., and Roughton, F. J. W., J. Physiol., 80, 113 (1934).
Scott, D. A., and Fisher, A. M., Trans. Roy. Soc. Can., Sec. 5, 36, 45 (1942).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
SCOTT, D., FISHER, A. Carbonic Anhydrase. Nature 153, 711–712 (1944). https://doi.org/10.1038/153711b0
Issue Date:
DOI: https://doi.org/10.1038/153711b0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.