Abstract
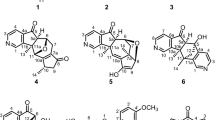
THE investigations of Ruzicka, and of Winterstein and their co-workers, have shown that the majority of the triterpene acids (hederagenin, gypsogenin, oleanolic acid, sia- and suma-resinolic acids) are in all probability θ-hydroxy-γ: δunsaturated mono-basic acids, as indicated in the accompanying formula1. Onthe other hand, β-boswellinic acid, C29H46(OH)COOH, one of the principal triterpene constituents of frankincense, evidently possesses a somewhat different constitution, for on mild oxidation with chromic anhydride this acid is converted into a monoketone C29H46O, m.p. 196° (found: C, 84·53; H, 11·11. C29H46O requires C, 84·80 ; H, 11·30 per cent), which has been characterized by the preparation of an oxime. Oxidation under similar conditions of β-boswellinic methyl ester, however, furnishes the corresponding keto-ester, m.p. 160° (found: C, 79·12 ; H, 10·20. C31H48O3 requires C, 79·41 ; H, 10·33 per cent), which forms an oxime, m.p. 200°.
Similar content being viewed by others
Article PDF
References
Ruzicka, Goldberg, and Hofmann, Helv. chim. Acta, 20, 325 (1937).
Jacobs and Gustus, J. Biol. Chem., 69, 641 (1926).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
SIMPSON, J. Structure of -Boswellinic Acid. Nature 140, 467 (1937). https://doi.org/10.1038/140467a0
Issue Date:
DOI: https://doi.org/10.1038/140467a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.