Abstract
This study examined the effects of a nonselective opiate antagonist and antagonists selective for the μ1 versus δ opioid receptor on ethanol-seeking behavior induced by alcohol-related environmental stimuli in an animal model of relapse. Rats were trained to self-administer ethanol (10% w/v) or water on an FR 1 schedule in 30-min daily sessions. The availability of ethanol was signaled by an olfactory discriminative stimulus (S+). A different olfactory stimulus (S−) signaled water availability. In addition, each lever-response resulting in delivery of ethanol was paired with illumination of a visual cue for 5 s (SC+), whereas a 5-s white noise (SC−) was associated with water. The rats were then subjected to a 20-day extinction phase where lever presses had no programmed consequences. Reexposure to the S+/CS+ stimulus condition in the absence of further ethanol availability elicited strong recovery of responding. No effect was observed following presentation of S−/CS−. Subsequentely, ethanol-seeking behavior associated with the S+/CS+ stimulus condition was studied in rats treated with the nonselective opiate antagonist naltrexone (0.25–1 mg/kg, SC), the δ selective antagonist naltrindole (1–5 mg/kg, IP), and the μ1 selective antagonist naloxonazine (1–15 mg/kg, IP). Naltrexone (1 mg/kg) and naltrindole (5 mg/kg) selectively inhibited alcohol-seeking behavior. Naloxonazine (15 mg/kg) also reduced ethanol-seeking behavior but produced some nonselective behavioral suppression as well. The results provide evidence that selective blockade of either μ1 or δ opioid receptors inhibits ethanol-seeking behavior elicited by drug-related environmental stimuli. Moreover, the data suggest that drugs aimed at the δ opioid receptor may offer advantages in the treatment and prevention of relapse compared with agents that also block the μ1 receptor.
Similar content being viewed by others
Main
Opiate antagonists inhibit the reinforcing effects of ethanol in a variety of animal models suggesting that endogenous opioid systems participate in the regulation of alcohol-seeking behavior and use. For example, opiate receptor antagonists reduce home cage voluntary ethanol intake as well as operant ethanol self-administration (Altshuler et al. 1980; Samson and Doyle 1985; Froehlich et al. 1990; Weiss et al. 1990; Hubbell et al. 1991; Kornet et al. 1991; Hyytia¨ and Sinclair, 1993; Davidson and Amit, 1997) and block ethanol-induced conditioned place preference (Cunningham and Prather 1992; Middaugh and Bandy 2000). In addition, the nonselective opiate receptor antagonist naltrexone has been shown to reduce ethanol consumption and relapse rates effectively among abstinent alcoholics in treatment (O'Malley et al. 1992; Volpicelli et al. 1992; O'Brien et al. 1996).
Opioid mechanisms appear to be involved not only in the direct reinforcing effects of ethanol, but also in the regulation of the appetitive effect of ethanol. This is suggested by studies showing that naltrexone reduces the urge to drink elicited by presentation of alcohol cues in human alcoholics (Monti et al. 1999; Rohsenow et al. 2000), and decreases the efficacy of an alcohol cue to reinstate extinguished responding at a previously drug-paired lever in rats (Katner et al. 1999). These observations suggest that naltrexone may blunt the motivating effects of ethanol-related environmental stimuli. Such an action would seem to represent a highly important aspect of the pharmacotherapeutic potential of naltrexone or other opiate antagonists in light of evidence that subjective reactions resulting from the conditioning of ethanol's effects with environmental stimuli are a potentially critical factor in relapse risk following detoxification and abstinence (Ludwig et al. 1974; Staiger and White 1991; O'Brien et al. 1992; Robbins and Ehrman 1992; Robbins et al. 1992; Childress et al. 1993).
Among the opiate receptor families, all three major receptor types (i.e., μ, δ, and κ) have been implicated in the regulation of both unconditioned and conditioned behavioral effects of ethanol. Selective μ or δ opioid receptor antagonists block ethanol-induced conditioned place preference and suppress the self-administration of ethanol in rats (Cunningham and Prather 1992, Hyytia¨ 1993; Krishnan-Sarin et al. 1995a; 1995b; Honkanen et al. 1996; Krishnan-Sarin et al. 1998; Matsuzawa et al. 1998). Selective κ antagonists, on the other hand, exert an opposite effect and facilitate the expression of conditioned ethanol place preference (Matsuzawa et al. 1999). Thus, existing evidence suggests that stimulation of μ and/or δ opioid receptors is linked to the positive reinforcing effects of ethanol, whereas activation of the κ opioid receptor family mediates aversive and dysphoric aspects of ethanol's actions. Little information is available, however, about the involvement of specific opioid receptor types in the control of alcohol-seeking behavior induced by ethanol-related environmental stimuli. Although naltrexone can inhibit ethanol craving and ethanol-seeking associated with exposure to ethanol cues (Katner et al. 1999; Monti et al. 1999) it is not known whether this effect requires blockade of both μ or δ receptors or whether μ or δ receptors play a selective role in conditioned responses to ethanol-associated stimuli. The objective of the present study was, therefore, to evaluate the effects of antagonists selective for the μ1 versus δ opioid receptor on ethanol-seeking behavior induced by alcohol-related environmental stimuli in a rat model of relapse (Katner et al. 1999), and to compare the effects of these agents with those produced by naltrexone. For this purpose, in the present study we used naloxonazine and naltrindole that selectively bind to μ1 and δ opioid receptors, respectively (Ling et al. 1986; Cruciani et al. 1987; Portoghese et al. 1988a;1988b).
METHODS
Subjects
Twenty-four male Wistar rats (Charles River Co., Kingston, NY) weighing 150–180 g at the beginning of the experiments were used. Rats were housed in groups of three in a temperature and humidity controlled vivarium on a reverse 12-hour light/dark cycle (on, 6:00 P.M.; off, 6:00 A.M.). All training and experimental sessions were conducted during the dark phase of the cycle. Standard laboratory rat chow and water were available ad libitum in the home cage, except as noted in “Oral Ethanol Self-Administration Training” (see below). All experimental procedures were carried out in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Drugs
Naltrexone hydrochloride (Sigma, St. Louis, MO) was dissolved in saline and was injected subcutaneously (SC) 30 min before the reinstatement tests. The selective δ opioid receptor antagonist naltrindole hydrochloride and the selective μ antagonist naloxonazine hydrochloride (RBI, Research Biomedical International, Natick, MA) were dissolved in distilled water and administered intraperitoneally (IP) 15 min (naltrindole) or 20 h (naloxonazine) before the reinstatement tests.
Oral Ethanol Self-Administration Apparatus
The self-administration stations consisted of operant conditioning chambers (Coulborn Instruments, Allentown, PA) enclosed in sound-attenuating, ventilated environmental cubicles. Each chamber was equipped with a drinking reservoir (vol. capacity: 0.15 ml) positioned 4 cm above the grid floor in the center of the front panel of the chamber, and 2 retractable levers, located 3 cm (1 to the right and the other to the left) of the drinking receptacle. Auditory and visual stimuli were presented via a speaker and a light located on the front panel. An IBM compatible microcomputer controlled the delivery of fluids, presentation of auditory and visual stimuli, and recording of behavioral data.
Self-Administration Training
Animals were trained to self-administer 10% ethanol or water in 30-min daily sessions on an FR 1 schedule of reinforcement where each response resulted in delivery of 0.1 ml of fluid as previously described (Weiss et al. 1993). Briefly, for the first 3 days of training, the rats were placed on a restriction schedule limiting water availability to 2 h/day in order to facilitate acquisition of operant responding maintained by a liquid reinforcer. During this time, responses at the lever were reinforced by delivery of a 0.2% (w/v) saccharin solution into the drinking receptacle. During all subsequent training and testing, water was freely available in the home cages. Following acquisition of saccharin-reinforced responding, rats were trained to self-administer ethanol using a modification of the sucrose-fading procedure (Samson 1986) that employed saccharin instead of sucrose (Weiss et al. 1993). During the first 6 days of training, responses at the lever were reinforced by a 0.2% saccharin (w/v) solution containing 5.0% (w/v) ethanol. After acquisition of saccharin-maintained responding, a second but inactive lever was introduced. During all training and testing phases, responses at this lever were recorded as a measure of nonspecific behavioral activation but had no programmed consequences. Starting on day 7, the concentration of ethanol was gradually increased from 5.0% to 8.0% and finally 10% (w/v), while the concentration of saccharin was correspondingly decreased to 0%.
Conditioning Phase
Beginning with self-administration training at the 10% ethanol concentration, olfactory discriminative stimuli (SD) predictive of ethanol versus water availability were presented just before and during the ethanol and water self-administration sessions. The olfactory stimuli were generated by depositing 6 drops of discrete food flavor extracts (Schilling®, Sparks, MD) into the bedding of the operant conditioning chamber. A banana flavor extract served as an S+ to signal the availability of ethanol. Water availability (i.e., nonreward) was signaled by the odor of an anise extract (S−). In addition, each lever-press resulting in delivery of ethanol was paired with illumination of the chamber's house light for 5 s (CS+), whereas a 5-s white noise (CS−) was presented response-contingently during water self-administration sessions. During the presentation of the CS+ or CS−, a 5-s time-out period was in effect, during which responses were recorded but not reinforced. The olfactory discriminative stimuli were introduced 1 minute before extension of the lever into the chamber and remained present throughout the 30-min ethanol or water self-administration sessions. The bedding was changed and bedding trays were thoroughly cleaned between sessions. During the first 3 days of the conditioning procedure, only ethanol training sessions were conducted. Subsequently, ethanol and water sessions were conducted in random order across training days with the constraint that all rats received a total of 10 ethanol and 10 water sessions.
Extinction Phase
At the end of the conditioning phase, rats were subjected to daily 30-min extinction sessions for a total of 20 days. During this phase, sessions began by extension of the levers without presentation of the discriminative stimuli (banana or anise odors). Responses at the previously active lever activated the syringe pump motor but did not result in delivery of ethanol, water, or presentation of the response-contingent cues (house light or white noise).
Reinstatement Testing
Reinstatement tests began 1 day after the final extinction session. These tests lasted 30 min and were conducted under conditions identical to those during the conditioning phase of the experiment, except that ethanol or water was not made available. Sessions were initiated by extension of both levers and presentation of either the ethanol- (S+) or nonreward-associated (S−) SD. The respective SD remained present during the entire session. Responses at the previously active lever were followed by activation of the syringe pump motor and a 5-s presentation of the CS+ (house light) in the S+ condition, or presentation of the CS− (white noise) in the S- condition. Half of the animals were tested under the S+/CS+ condition on day 1 and the S−/CS− condition on the subsequent day. The remaining animals were tested under the S−/CS− condition on day 1, followed by testing under S+/CS+ conditions on day 2.
For testing of the effects of opiate receptor antagonists, rats were matched on the basis of their performance during the S+/CS+ reinstatement test and divided into three groups with the same mean number of reinstatement responses. Four days after completion of the initial reinstatement tests, rats were treated with naltrexone (0.0, 0.25–1.0 mg/kg), naloxonazine (0.0, 5.0–15.0 mg/kg), and naltrindole (0.0, 1.0–5.0 mg/kg) according to a Latin square design. The opiate antagonists were tested first for their effects on responding under the S+/CS+ condition, and at completion of this experiments rats were tested again under the S−/CS− condition. Drug tests were separated by 3-day intervals during which the animals remained confined to their home cages in the vivarium.
Statistical Analysis
Responses during the conditioning phase were analyzed by a two-way analysis of variance (ANOVA) with repeated measures, one factor being time (days) and the other factor being treatment (water or ethanol). The remaining data were analyzed by one-way analysis of variance (ANOVA) with repeated measures. Significant differences among individual treatment conditions were determined by Newman-Keuls post-hoc comparisons.
RESULTS
Discrimination Phase
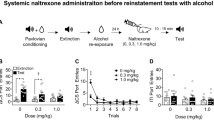
Following acquisition of reliable ethanol (10%) self-administration at the end of the saccharin fading procedure, ethanol-reinforced responding increased progressively during the conditioning phase. At the same time, lever responses during water self-administration sessions decreased slightly. After 20 training days (i.e., 10 ethanol and 10 water sessions), the rats developed stable levels of responding. The mean (± SEM) number of responses over the last three sessions of the conditioning phase were 39.59 ± 1.75 during ethanol sessions (corresponding to 0.67 ± 0.1 g/kg of ethanol) and 25.40 ± 0.43 during water sessions (Figure 1). Previous studies demonstrated that self-administration of ethanol over this range of doses lead to a pharmacologically relevant blood ethanol concentration in rats (Roberts et al. 1999). ANOVA confirmed an overall significant difference between “drinking solutions” (i.e., ethanol and water) [F(1,23) = 5.21 p < .05] and a significant interaction between “drinking solution” and “training days” [F(9,207) = 6.81 p < .001]. Post-hoc comparisons indicated that responding in the ethanol versus water condition differed significantly starting with day 7 of the conditioning phase. The number of responses at the inactive lever ranged between 6.7 ± 1.2 of the first day to 3.4 ± 0.8 of the last day of discrimination, and statistical analysis did not reveal any significant differences between water and ethanol sessions [F(1,23) = 2.81 NS].
Lever responses in 30-min sessions during the conditioning, extinction, and reinstatement phases in rats (n = 24). Conditioning phase: Mean (± SEM) number of responses during ethanol (EtOH) and water self-administration sessions. Data represent responses during the final respective session in this experimental phase. Extinction phase: Mean (± SEM) number of responses at the previously active lever during the 20th and final extinction session. Reinstatement phase: Mean (± SEM) number of responses at the previously active lever under the stimulus conditions associated with ethanol (S+/CS+) or non-reward/water (S−/CS−). ** p < .01; different from extinction responses and performance.
Extinction Phase
At previously active lever, rats emitted an average (± SEM) of 28.8 ± 2.8 responses during the first extinction session. During subsequent sessions, lever pressing progressively decreased, and over the last three extinction sessions, the mean (± SEM) number of responses was 6.79 ± 0.86 (Figure 1). At the inactive lever, responses remained very low for the entire period of extinction and ranged from 6.46 ± 1.8 measured the 4th day of extinction to 2.86 ± 0.6 of the last extinction day.
Reinstatement Phase
Exposure to the S+/CS+ stimulus condition during the initial reinstatement test elicited immediate recovery of responding with a mean (± SEM) increase in the number of responses at the previously active lever from 6.79 ± 0.86 (extinction) to 24.10 ± 2.7 (p < .01). Conversely, exposure to the S-/CS- stimulus conditions previously associated with water availability did not alter responding compared with extinction levels (Figure 1). The difference in responding between the S+/CS+ and S−/CS− conditions was confirmed by a significant main effect of “stimulus condition” [F(2,23) = 44.66 p < .001]. The number of responses at the inactive lever were 2.86 ± 0.6 the last day of extinction, and 2.6 ± 0.8 or 3.1 ± 1.3 following the reinstatement under S+/CS+ or S−/CS− conditions, respectively. Statistical analysis did not reveal any significant effect at the inactive lever [F(2,23) = 0.63 NS].
During the initial reinstatement test, one animal failed to respond in the S+/CS+ condition and was therefore excluded from the subsequent pharmacological tests with opiate antagonists. This animal had been designated for testing with naltrexone. In addition, in the group of rats tested with naltrindole, one animal developed health problems and did not complete the dose-response determination. Data from this animal were therefore not used for statistical analysis. Thus, the remaining final sample sizes for the three drug groups were n = 7 (naltrexone), n = 7 (naltrindole), and n = 8 (naloxonazine).
All three opiate antagonists attenuated ethanol-seeking behavior in the S+/CS+ condition. Naltrexone significantly (p < .05) decreased responding at the 1 mg/kg dose [Newman-Keuls following overall ANOVA: F(2,6) = 4.09 p < .05]. Specifically, at this dose, lever-pressing decreased by approximately 60% from 16.57 ± 2.05 (vehicle) to 7.00 ± 2.14 (Figure 2A). Conversely, naltrexone did not alter responding in the S−/CS− condition at any dose [F(2,6) = 3.07; NS].
Mean (± SEM) number of responses during 30-min reinstatement test sessions following pretreatment with (A) naltrexone (n = 7), (B) naltrindole (n = 7), and (C) naloxonazine (n = 8). For each drug, rats were tested under the stimulus conditions previously associated with ethanol (S+/CS+) or non-reward/water (S−/CS−) according to a Latin square design. * p < .05; significantly different from vehicle (Veh) treatment.
The δ-opioid antagonist naltrindole also attenuated responding in the S+/CS+ condition [F(2,6) = 4.58 p < .05]. Post-hoc comparisons indicated that the inhibition of responding was significant at the highest dose (5 mg/kg) of the drug (p < .05). Responding at this dose was reduced by about 50% from 12.57 ± 2.30 (vehicle) to 7.28 ± 1.89 (Figure 2B). In contrast, naltrindole did not modify lever-pressing behavior in the S−/CS− test condition [F(2,6) = 0.21; NS].
In the case of the μ1 antagonist, naloxonazine, a significant overall effect of treatment was observed under both the S+/CS+ [F(2,7) = 4.22 p < .05] and S−/CS− [F(2,6) = 4.58 p < .05] test conditions. Following treatment with 15 mg/kg of naloxonazine, S+/CS+-associated responses were significantly (p < .05) reduced by about 55% from 14.63 ± 2.89 (vehicle) to 6.82 ± 2.41 (Figure 2C). However, at this dose, an even greater suppression of behavior was observed in the S−/CS− stimulus condition (p < .05) where responding was reduced by 66% from 9.00 ± 1.36 (vehicle) to 3.00 ± 1.10.
During the various drug testing, the number of lever presses measured at the inactive lever was extremely low and was not influenced by the different drug treatments. This is confirmed by the analysis of variance that revealed no significant effect following naltrexone [F(2,6) = 0.96 NS], naltrindole [F(2,6) = 0.33 NS], and naloxonazine [F(2,7) = 3.28 NS].
DISCUSSION
The results confirm that environmental stimuli associated with the subjective effects of ethanol and/or predictive of ethanol availability reliably reinstate responding at the previously active, ethanol-paired lever (Katner et al. 1999; Katner and Weiss, 1999; Ciccocioppo et al. 2001). Lever-responses in the S−/CS− condition remained at extinction levels and the number of responses at the previously inactive lever was negligible under both the S+/CS+ and S−/CS− stimulus conditions. These observations rule out nonspecific arousal as a factor in the response reinstatement, and confirm that the animals’ behavior was controlled selectively by the ethanol-associated stimuli.
Pretreatment with both a nonselective opiate receptor antagonist and μ- or δ-selective antagonists significantly attenuated ethanol-seeking behavior in the S+/CS+ condition. These agents differed, however, in the degree to which they produced nonselective behavioral effects as measured by suppression of responding in the S−/CS− condition. In the case of naltrexone, the attenuation of ethanol-seeking behavior at the effective 1 mg/kg dose was selective, and lever-pressing associated with exposure to the stimuli previously associated with water was not significantly modified. Similar results with naltrexone, albeit at a lower effective dose (0.25 mg/kg), have been reported in a previous study that employed ethanol odor as an olfactory discriminative stimulus for drug availability to elicit conditioned ethanol-seeking behavior (Katner et al. 1999). Naltrexone is a nonselective opioid antagonist with high affinity for μ, δ, and κ receptors. Therefore, an important objective of this study was to investigate the effect of selective blockade of specific opioid receptor subtypes on the reinstatement of ethanol-seeking behavior. For this purpose, the selective μ1 opioid receptor antagonist naloxonazine and the selective δ antagonist naltrindole were employed.
Naltrindole, at a dose of 5 mg/kg selectively reduced ethanol-seeking behavior in the S+/CS+ condition without affecting responding in the S−/CS− condition. At the doses used here, naltrindole has been shown to attenuate a variety of behaviors that are selectively mediated by δ opioid receptors (Hart et al. 1983; Kitchen and Pinker 1990; Dhawan et al. 1996). Behaviors involving an action at other opioid receptors are modified by naltrindole only at higher doses. For instance, the discriminative stimulus effects of morphine, which are mediated by the μ receptor, are not blocked by naltrindole at doses as high as 10 mg/kg (Stevenson et al. 2000). In addition, in vitro studies have demonstrated that naltrindole has nanomolar affinity for δ receptors while it binds μ and κ receptors at more than 100 times higher concentrations (Rogers et al. 1990).
Inhibition of S+/CS+-induced responding at the previously active lever was also seen following administration of the selective μ1 receptor antagonist naloxonazine (15 mg/kg). The effect of the drug was not specific, however, in that the lowest effective dose that attenuated ethanol-seeking behavior also suppressed responses below baseline levels in the S−/CS− control condition. Naloxonazine was used at doses that have been shown to block μ1 receptors selectively without affecting the activity of other opioid receptor subtypes (Dhawan et al. 1996). Because naloxonazine blocks central μ1 opioid receptors irreversibly and, thus, exerts antagonist effects at these sites in excess of 24 h, the drug was administered 20 h before testing. Therefore, the time lag chosen between administration of the drug and the reinstatement test was well within the window of μ1 antagonist activity. At the same time, the time lag minimized possible effects of naloxonazine produced by the initial reversible blockade produced by this compound of other opioid receptors, including the μ2 opioid receptor subtype (Ling et al. 1986; Johnson and Pasternak 1984).
In another work conducted in our laboratory, it was shown that during the first reinstatement test responding at the previously ethanol-paired lever is around 30% higher compared with the subsequent reinstatement tests (Ciccocioppo et al. 2001). During the following tests, however, the level of responding stabilized and did not decrease over testing (Ciccocioppo et al. 2001). This suggests that, in the present study, it is unlikely that the effect of the drugs was influenced by the repeated testing procedure adopted, because no spontaneous decrease of responding should have occurred over the drug treatments initiated after the first reinstatement test. Finally, the possibility that the low level of responding under S−/CS− could have generated a “floor effect” and limited the sensitivity to detect a significant drug effect under this condition contradicts previously published data (Katner et al. 1999). In effect, Katner and co-workers, using a slightly different reinstatement procedure, demonstrated the efficacy of naltrexone to inhibit eve-induced ethanol seeking behavior when the level of responding was approximately the same (6–8 responses) as that observed for water-paired cues in the present study.
There is ample evidence that ethanol stimulates the activity of the endogenous opioid β-endorphin and enkephalin systems, and that the rewarding effects of ethanol are, at least in part, mediated via activation of brain μ1 and δ opioid receptors by these neuropeptides (Herz 1997). It has also consistently been shown that treatments with the selective μ1/μ2 receptor antagonists β-funaltrexamine and CTOP, the μ1 antagonist naloxonazine, or the selective δ receptor antagonists naltriben and naltrindole reduce ethanol consumption in rodents (Froehlich et al. 1991; Hyytiä 1993; Krishnan-Sarin et al. 1995a, 1995b, 1998; Honkanen et al. 1996). In addition, a recent place conditioning study demonstrated that blockade of μ or δ receptors by β-funaltrexamine or naltrindole significantly attenuates ethanol- induced conditioned place preference (Matsuzawa et al. 1999). Thus, several lines of evidence implicate both μ1 and δ opioid receptors in the mediation of the rewarding effect of ethanol. Considering the results of the present study, μ1 and δ opioid receptors may not only play a prominent role in ethanol reward, but also in the motivating effects of alcohol-associated environmental stimuli.
The mechanism by which opioid antagonists inhibit the behavioral response to ethanol-related stimuli is unclear. Evidence is accumulating that one mode of action by which opiate antagonists reduce the primary reinforcing effects of ethanol in rats self-administering alcohol is by interfering with ethanol-dependent dopaminergic activation (Gonzales and Weiss 1998; Acquas et al. 1993; Benjamin et al. 1993). It is possible that reversal of the motivating effects of ethanol-related stimuli by opiate antagonists may involve an opioid-dopamine link as well. This hypothesis is supported by two pieces of evidence. First, exposure to drug-associated contextual stimuli can increase dopamine release in the nucleus accumbens (Weiss et al. 1993; Katner and Weiss, 1999; Weiss et al. 2000). Moreover, treatment with a selective dopamine D1 receptor antagonist reduces ethanol-seeking behavior elicited by an alcohol-predictive discriminative stimulus (Liu and Weiss, unpublished observations). Second, it is known that δ and μ1 opioid receptors have a tonic modulatory role on mesolimbic DA neurons projecting from the ventral tegmental area to the nucleus accumbens, and that stimulation of these opioid receptors facilitates dopamine transmission in this pathway (Devine et al. 1993; Di Chiara and North, 1992). Therefore, blockade of opioid receptors by selective δ or μ1 may result in an inhibition of the meso-accumbal DA neurotransmission which, in turn, may reduce the ability of cues to reinstate ethanol-seeking behavior.
It is important, however, to consider that the opioidergic modulation of DA neurons in the ventral tegmental area involves predominantly μ1 receptors (Tanda and Di Chiara 1998), whereas the modulation of DA terminal activity is predominantly under control of the δ receptor subtype (Borg and Taylor 1997). The different localization of these receptor subtypes therefore may suggest that they can modulate DA activity by distinct mechanisms. Specifically, the μ1 receptor antagonists may reduce the ability of cues to increase the firing rate of ventral tegmental area dopamine neurones that project to the nucleus accumbens, whereas selective δ opioid receptor antagonists may exert their effect by inhibiting DA release from axon terminals located in the accumbens. Nevertheless, the results of the present study demonstrate that both classes of selective antagonists potently inhibit the reinstatement of ethanol-seeking behavior. Therefore, one may speculate that both mechanisms may contribute to the effective anti-relapse actions of nonselective opioid receptor antagonists such as naltrexone. On the other hand, the results demonstrate that selective blockade of δ opioid receptors by naltrindole produced an effective and selective inhibition of ethanol-seeking behavior. Interestingly, conditioned taste aversion studies in rats demonstrated that selective blockade of δ opioid receptors results in less aversive effects compared with those observed after administration of nonselective or selective μ opioid antagonists (Parker and Rennie 1992; Hutchinson et al. 2000). Together, these observations suggest that the δ opioid receptor may represent a promising target for the development of treatment medications for alcohol craving and relapse.
In conclusion, the results provide evidence for an involvement of both δ and μ1 opioid receptors in the motivating effects of ethanol-associated environmental stimuli. These observations are consistent with clinical findings demonstrating that the opioid antagonist naltrexone is effective in the treatment of alcohol craving and relapse in humans (O'Malley et al. 1992; Volpicelli et al. 1992; Volpicelli et al. 1995). Moreover, the results suggest that drugs aimed at the δ opioid receptor may offer advantages in the treatment and prevention of relapse compared with agents that also block the μ1 receptor.
References
Acquas E, Meloni M, Di Chiara G . (1993): Blockade of δ-opioid receptors in the nucleus accumbens prevents ethanol-induced stimulation of dopamine release. Eur J Pharmacol 230: 239–241
Altshuler HL, Phillips PE, Feinhandler DA . (1980): Alteration of ethanol self-administration by naltrexone. Life Sci 26: 679–688
Benjamin D, Grant ER, Pohorecky LA . (1993): Naltrexone reverses the ethanol-induced dopamine release in the nucleus accumbens in awake, freely moving rats. Brain Res 621: 137–140
Borg PJ, Taylor DA . (1997): Involvement of mu- and delta-opioid receptors in the effects of systemic and locally perfused morphine on extracellular levels of dopamine, DOPAC and HVA in the nucleus accumbens of the halothane- anaesthetized rat. Naunyn Schmiedebergs Arch Pharmacol 355: 582–588
Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O'Brien CP . (1993): Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr 137: 73–95
Ciccocioppo R, Angeletti S, Weiss F . (2001): Long-lasting resistance to extinction of response reinstatement induced by ethanol-related stimuli: Role of genetic ethanol preference. Alcohol Clin Exp Res 25: 1414–1419
Cruciani RA, Lutz RA, Munson PJ, Rodbard D . (1987): Naloxonazine effects on the interaction of enkephalin analogs with mu-1, mu and delta opioid binding sites in rat brain membranes. J Pharmacol Exp Ther 242: 15–20
Cunningham CL, Prather LK . (1992): Conditioning trial duration affects ethanol-induced conditioned place preference in mice. Anim Learn Behav 20: 187–194
Davidson D, Amit Z . (1997): Naltrexone blocks acquisition of voluntary ethanol intake in rats. Alcohol Clin Exp Res 21: 677–683
Devine DP, Leone P, Pocock D, Wise RA . (1993): Differential involvement of ventral tegmental mu, delta and kappa opioid receptors in modulation of basal mesolimbic dopamine release: In vivo microdialysis studies. J Pharmacol Exp Ther 266: 1236–1246
Di Chiara G, North RA . (1992): Neurobiology of opiate abuse. Trends Pharmacol Sci 13: 185–193
Dhawan BN, Cesselin F, Raghubir R, Reisine T, Bradley PB, Portoghese PS, Hamon M . (1996): International Union of Pharmacology. XII. Classification of opioid receptors. Pharmacol Rev 48: 567–592
Froehlich JC, Harts J, Lumeng L, Li TK . (1990): Naloxone attenuates voluntary ethanol intake in rats selectively bred for high ethanol preference. Pharmacol Biochem Behav 35: 385–390
Froehlich JC, Zweifel M, Harts J, Lumeng L, Li TK . (1991): Importance of delta opioid receptors in maintaining high alcohol drinking. Psychopharmacology (Berl) 103: 467–472
Gonzales RA, Weiss F . (1998): Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J Neurosci 18: 10663–10671
Hart SL, Slusarczyk H, Smith TW . (1983): The involvement of opioid delta-receptors in stress induced antinociception in mice. Eur J Pharmacol 95: 283–285
Herz A . (1997): Endogenous opioid systems and alcohol addiction. Psychopharmacology (Berl) 129: 99–111
Honkanen A, Vilamo L, Wegelius K, Sarviharju M, Hyytia P, Korpi ER . (1996): Alcohol drinking is reduced by a mu 1-but not by a delta-opioid receptor antagonist in alcohol-preferring rats. Eur J Pharmacol 304: 7–13
Hubbell CL, Marglin SH, Spitalnic SJ, Abelson ML, Wild KD, Reid LD . . (1991): Opioidergic, serotonergic, and dopaminergic manipulations and rats′ intake of a sweetened alcoholic beverage. Alcohol 8: 355–367.
Hutchinson AC, Simpson GR, Randall JF, Zhang X, Calderon SN, Rice KC, Riley AL . (2000): Assessment of SNC 80 and naltrindole within a conditioned taste aversion design. Pharmacol Biochem Behav 66: 779–787
Hyytiä P . (1993): Involvement of mu-opioid receptors in alcohol drinking by alcohol-preferring AA rats. Pharmacol Biochem Behav 45: 697–701
Hyytiä P, Sinclair JD . (1993): Responding for oral ethanol after naloxone treatment by alcohol-preferring AA rats. Alcohol Clin Exp Res. 17: 631–636
Johnson N, Pasternak GW . (1984): Binding of [3H]naloxonazine to rat brain membranes. Mol Pharmacol 26: 477–483
Katner SN, Magalong JG, Weiss F . (1999): Reinstatement of alcohol-seeking behavior by drug-associated discriminative stimuli after prolonged extinction in the rat. Neuropsychopharmacology 20: 471–479
Katner SN, Weiss F . (1999): Ethanol-associated olfactory stimuli reinstate ethanol-seeking behavior after extinction and modify extracellular dopamine levels in the nucleus accumbens. Alcohol Clin Exp Res 23: 1751–1760
Kitchen I, Pinker SR . (1990): Antagonism of swim-stress-induced antinociception by the delta-opioid receptor antagonist naltrindole in adult and young rats. Br J Pharmacol 100: 685–688
Kornet M, Goosen C, Van Ree JM . (1991): Effect of naltrexone on alcohol consumption during chronic alcohol drinking and after a period of imposed abstinence in free-choice drinking rhesus monkeys. Psychopharmacology (Berl) 104: 367–376
Krishnan-Sarin S, Jing SL, Kurtz DL, Zweifel M, Portoghese PS, Li TK, Froehlich JC . (1995a): The delta opioid receptor antagonist naltrindole attenuates both alcohol and saccharin intake in rats selectively bred for alcohol preference. Psychopharmacology (Berl) 120: 177–185
Krishnan-Sarin S, Portoghese PS, Li TK, Froehlich JC . (1995b): The delta 2-opioid receptor antagonist naltriben selectively attenuates alcohol intake in rats bred for alcohol preference. Pharmacol Biochem Behav 52: 153–159
Krishnan-Sarin S, Wand GS, Li XW, Portoghese PS, Froehlich JC . (1998): Effect of mu opioid receptor blockade on alcohol intake in rats bred for high alcohol drinking. Pharmacol Biochem Behav 59: 627–635
Ling GS, Simantov R, Clark JA, Pasternak GW . (1986): Naloxonazine actions in vivo. Eur J Pharmacol 129: 33–38
Ludwig AM, Wikler A, Stark LH . (1974): The first drink. Psychobiological aspects of craving. Arch Gen Psychiatry 30: 539–547
Matsuzawa S, Suzuki T, Misawa M, Nagase H . (1998): Involvement of mu- and delta-opioid receptors in the ethanol-associated place preference in rats exposed to foot shock stress. Brain Res 803: 169–177
Matsuzawa S, Suzuki T, Misawa M, Nagase H . (1999): Different roles of mu-, delta- and kappa-opioid receptors in ethanol-associated place preference in rats exposed to conditioned fear stress. Eur J Pharmacol 368: 9–16
Middaugh LD, Bandy AL . (2000): Naltrexone effects on ethanol consumption and response to ethanol conditioned cues in C57BL/6 mice. Psychopharmacology (Berl) 151: 321–327
Monti PM, Rohsenow D J, Hutchison K E, Swift R M, Mueller T I, Colby S M, Brown RA, Gulliver SB, Gordon A, Abrams DB . (1999): . Naltrexone's effect on cue-elicited craving among alcoholics in treatment. Alcohol Clin Exp Res 23: 1386–1394
O'Brien C, Childress AR, Ehrman R, Robbins S, McLellan AT . (1992): Conditioning mechanisms in drug dependence. Clin Neuropharmacol 15(Suppl 1, Pt A): 66A–67A
O'Brien CP, Volpicelli LA, Volpicelli JR . (1996): Naltrexone in the treatment of alcoholism: A clinical review. Alcohol 13: 35–39
O'Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B . (1992): Naltrexone and coping skills therapy for alcohol dependence. A controlled study. Arch Gen Psychiatry 49: 881–887
Parker LA, Rennie M . (1992): Naltrexone-induced aversions: Assessment by place conditioning, taste reactivity, and taste avoidance paradigms. Pharmacol Biochem Behav 41: 559–565
Portoghese PS, Sultana M, Nagase H, Takemori AE . (1988a): Application of the message-address concept in the design of highly potent and selective non-peptide delta opioid receptor antagonists. J Med Chem 31: 281–282
Portoghese PS, Sultana M, Takemori AE . (1988b): Naltrindole, a highly selective and potent non-peptide delta opioid receptor antagonist. Eur J Pharmacol 146: 185–186
Robbins SJ, Ehrman RN . (1992): Designing studies of drug conditioning in humans. Psychopharmacology (Berl) 106: 143–153
Robbins SJ, Ehrman RN, Childress AR, O'Brien CP . (1992): Using cue reactivity to screen medications for cocaine abuse: A test of amantadine hydrochloride. Addict Behav 17: 491–499
Roberts AJ, Heyser CJ, Koob GF . (1999): Operant self-administration of sweetened versus unsweetened ethanol: Effects on blood alcohol levels. Alcohol Clin Exp Res 23: 1151–1157
Rogers H, Hayes AG, Birch PJ, Traynor JR, Lawrence AJ . (1990): The selectivity of the opioid antagonist, naltrindole, for delta-opioid receptors. J Pharm Pharmacol 42: 358–359
Rohsenow DJ, Monti PM, Hutchison KE, Swift RM, Colby SM, Kaplan GB . (2000): Naltrexone's effects on reactivity to alcohol cues among alcoholic men. J Abnorm Psychol 109: 738–742
Samson HH, Doyle TF . (1985): Oral ethanol self-administration in the rat: Effect of naloxone. Pharmacol Biochem Behav 22: 91–99
Samson HH . (1986): Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res 10: 436–442
Staiger PK, White JM . (1991): Cue reactivity on alcohol abusers: Stimulus specificity and extinction of the response. Addict Behav 16: 211–221
Stevenson GW, Canadas F, Zhang X, Rice KC, Riley AL . (2000): Morphine discriminative control is mediated by the Mu opioid receptor. Assessment of delta opioid substitution and antagonism. Pharmacol Biochem Behav 66: 851–856
Tanda G, Di Chiara G . (1998): A dopamine-mu1 opioid link in the rat ventral tegmentum shared by palatable food (Fonzies) and non-psychostimulant drugs of abuse. Eur J Neurosci 10: 1179–1187
Volpicelli JR, Alterman AI, Hayashida M, O'Brien CP . (1992): Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry 49: 876–880
Volpicelli JR, Watson NT, King AC, Sherman CE, O'Brien CP . (1995): Effect of naltrexone on alcohol “high” in alcoholics. Am J Psychiatry 152: 613–615
Weiss F, Mitchiner M, Bloom FE, Koob GF . (1990): Free-choice responding for ethanol versus water in alcohol preferring (P) and unselected Wistar rats is differentially modified by naloxone, bromocriptine, and methysergide. Psychopharmacology (Berl) 101: 178–186
Weiss F, Lorang MT, Bloom FE, Koob GF . (1993): Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: Genetic and motivational determinants. J Pharmacol Exp Ther 267: 250–258
Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smith DL, Ben-Shahar O . (2000): Control of cocaine-seeking behavior by drug-associated stimuli in rats: Effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci USA 97: 4321–4326
Acknowledgements
This work is publication number 14027-NP from The Scripps Research Institute and was supported by NIAAA grant AA10531 (FW). The authors thank Miwe Arends for help with the preparation of the article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Please send reprint requests to: Friedbert Weiss, Ph.D., Department of Neuropharmacology (CVN-15), The Scripps Research Institute, La Jolla, CA 92037, USA. Fax: +1-858-784-7393, E-mail: bweiss@mail.scripps.edu
Rights and permissions
About this article
Cite this article
Ciccocioppo, R., Martin-Fardon, R. & Weiss, F. Effect of Selective Blockade of μ1 or δ Opioid Receptors on Reinstatement of Alcohol-Seeking Behavior by Drug-Associated Stimuli in Rats . Neuropsychopharmacol 27, 391–399 (2002). https://doi.org/10.1016/S0893-133X(02)00302-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1016/S0893-133X(02)00302-0
Keywords
This article is cited by
-
Blocking μ-opioid receptors attenuates reinstatement of responding to an alcohol-predictive conditioned stimulus through actions in the ventral hippocampus
Neuropsychopharmacology (2023)
-
Learning processes in relapse to alcohol use: lessons from animal models
Psychopharmacology (2023)
-
Alcohol availability during withdrawal gates the impact of alcohol vapor exposure on responses to alcohol cues
Psychopharmacology (2022)
-
The effects of nalmefene on the impulsive and reflective system in alcohol use disorder: A resting-state fMRI study
Psychopharmacology (2022)
-
Translational opportunities in animal and human models to study alcohol use disorder
Translational Psychiatry (2021)