Abstract
We have previously shown that the risk of major depression in patients with malignant melanoma undergoing interferon-α (IFN-α) therapy can be reduced by pretreatment with the antidepressant, paroxetine. Using dimensional analyses, the present study assessed the expression and treatment responsiveness of specific clusters of neuropsychiatric symptoms over the first three months of IFN-α therapy.
Forty patients with malignant melanoma eligible for IFN-α treatment were randomly assigned to receive either paroxetine or placebo in a double-blind design. Neuropsychiatric assessments were conducted at regular intervals during the first twelve weeks of IFN-α therapy and included the 21-item Hamilton Depression Rating Scale, the 14-item Hamilton Anxiety Rating Scale and the Neurotoxicity Rating Scale.
Neurovegetative and somatic symptoms including anorexia, fatigue and pain appeared within two weeks of IFN-α therapy in a large proportion of patients. In contrast, symptoms of depressed mood, anxiety and cognitive dysfunction appeared later during IFN-α treatment and more specifically in patients who met DSM-IV criteria for major depression. Symptoms of depression, anxiety, cognitive dysfunction and pain were more responsive, whereas symptoms of fatigue and anorexia were less responsive, to paroxetine treatment. These data demonstrate distinct phenomenology and treatment responsiveness of symptom dimensions induced by IFN-α, and suggest that different mechanisms mediate the various behavioral manifestations of cytokine-induced “sickness behavior.”
Similar content being viewed by others
Main
Interferon-α (IFN-α) is a cytokine released early during viral infection and has both anti-viral and anti-proliferative properties. IFN-α has been used to treat a variety of chronic viral infections and malignant disorders and constitutes the only FDA-approved treatment for chronic hepatitis C, a viral infection that afflicts over 4 million individuals in the United States (Gutterman 1994; Kirkwood et al. 1996; Ravaud et al. 1999; Liang et al. 2000). Despite its potential therapeutic benefits, administration of IFN-α is frequently accompanied by the appearance of neuropsychiatric symptoms, e.g., depressed mood, anhedonia, anxiety and cognitive impairment, and neurovegetative and somatic symptoms, e.g., anorexia, fatigue, altered sleep, pain, and fever (Renault et al. 1987; Carpiniello et al. 1998; Valentine et al. 1998; Capuron et al. 2000). Clinical studies have shown that approximately 30 to 45% of patients receiving IFN-α develop depression during the course of therapy (Miyaoka et al. 1999; Musselman et al. 2001). The neuropsychiatric effects of IFN-α generally resolve after treatment cessation, but in some cases, residual symptoms persist for months (Meyers et al. 1991). IFN-α–induced neuropsychiatric toxicity is an important public health concern because it can reduce quality of life, compromise compliance, and lead to adjustment or interruption of IFN-α therapy, thus limiting its therapeutic potential.
A recent double-blind, placebo-controlled study in patients undergoing IFN-α therapy for the treatment of malignant melanoma indicated that pretreatment with the antidepressant paroxetine was effective in preventing the development of major depression and was associated with better adherence to IFN-α therapy (Musselman et al. 2001). The aim of the present study was to use dimensional analyses to examine the expression of specific clusters of neuropsychiatric and neurovegetative/somatic symptoms in these patients over the first 12 weeks of IFN-α treatment and determine the responsiveness of these symptoms to paroxetine administration.
METHODS
Patients
Forty patients (20 males, 20 females; age range: 25–74 years old) with resected malignant melanoma and a 50% or greater chance of recurrence participated in the study. This patient sample was identical to that reported in Musselman et al. (2001). Patients were recruited from the Winship Cancer Institute of the Emory University School of Medicine and were assigned to receive IFN-α therapy intravenously at the dose of 20 million international units (MIU) per square meter of body surface area five days per week for the first four weeks of therapy followed by 10 MIU/m2 subcutaneously three days per week for the remaining eight weeks of the study.
Patients with a diagnosis of schizophrenia or bipolar disorder as determined by the Structured Clinical Interview for DSM-IV (SCID) (American Psychiatric Association 1994), a Minimental State Exam ⩽ 24 (Folstein et al. 1975), or uncontrolled neurological, cardiovascular, endocrine, hematologic, hepatic or renal disease were excluded.
All patients were adults and provided signed informed consent before enrollment. The study was approved by the Human Investigations Committee of the Emory University School of Medicine.
Enrollment and Followup
After baseline evaluation, patients were randomly assigned in double-blind fashion to receive either placebo or paroxetine (Paxil) according to a permutation block randomization procedure.
Patients received study medication (paroxetine or placebo) two weeks before initiating IFN-α. The dosage of study medication was one tablet (10 mg of paroxetine per tablet) per day for the first week and two tablets per day for the second week. The dosage could be increased up to four tablets per day during the second week of IFN-α therapy.
Following baseline assessment, evaluations occurred on a weekly basis during the first four weeks of IFN-α therapy and every four weeks thereafter.
Assessments
At each evaluation, patients were administered a battery of observer-rated and self-report psychiatric assessments including the 21-item observer-rated Hamilton Rating Scale for Depression (HAM-D) (Hamilton 1960), the 14-item observer-rated Hamilton Anxiety Scale (HAM-A) (Maier et al. 1988) and the 39-item self-report Neurotoxicity Rating Scale (NRS) (Valentine et al. 1995). In addition, a semi-structured interview, a modified Structured Clinical Interview for DSM-IV (SCID), was administered by a psychiatrist to determine whether patients fulfilled symptom criteria for major depression. It should be noted that major depression occurring in association with IFN-α therapy is appropriately designated a “Substance-Induced Mood Disorder” in DSM-IV nomenclature. The HAM-D and HAM-A scales include items scored from 0–4 assessing the mood, somatic and cognitive symptoms of depression and anxiety. The NRS includes items scored from 0 (“not present”) to 4 (“extremely severe”) measuring the severity of various psychiatric, cognitive, neurovegetative and somatic symptoms known to be induced by cytokine therapy. Mean values of these scales as well as the development of major depression in this patient sample have been reported elsewhere (Musselman et al. 2001).
Dimensional Analyses
Four time points were chosen for the dimensional analyses because they allowed to capture immediate vs. delayed neurobehavioral effects of IFN-α: (1) baseline (before initiating IFN-α therapy or study medication); (2) two weeks following initiation of IFN-α treatment; (3) at the end of the second month of IFN-α treatment (week 8); and (4) at the end of the study (i.e., week 12).
To perform the dimensional analyses, neuropsychiatric and neurovegetative/somatic symptoms were grouped in four dimensions corresponding to mood, cognition, neurovegetative function and somatic symptoms respectively. The dimension of mood included depressive symptoms (depressed mood, feelings of guilt and suicidal thoughts assessed by the HAM-D scale and anhedonia assessed by the NRS) and anxious symptoms (anxious mood, tension/irritability, fear assessed by the HAM-A scale). The dimension of cognitive function included self-reported symptoms of memory impairment, distractibility, indecisiveness, word-finding problems and episodes of confusion as assessed by the NRS. The dimension of neurovegetative function included symptoms of anorexia, altered sleep (difficulty getting to sleep or staying asleep, sleeping too much), fatigue, and psychomotor retardation assessed by the NRS. Finally, the dimension of somatic symptoms included pain symptoms (headache, body aches, joint pain) and gastrointestinal distress (nausea, vomiting, bowel problems) assessed by the NRS. The mean score obtained by a patient in each dimension was calculated as the sum of the severity scores obtained by the patient in each of the symptoms in that dimension divided by the number of symptoms in that dimension. For example, the mean score obtained by a patient in the dimension of anxiety corresponded to the sum of the score on the three following items: anxious mood, tension/irritability, and fear, divided by three.
Statistical Analyses
Differences between placebo- and paroxetine-treated patients for categorial variables were assessed using the chi-square test. Analysis of Variance (ANOVA) with treatment (placebo vs. paroxetine) as the independent factor and time as the repeated measure (RM) factor were performed for mean comparisons. Post hoc comparisons were made using both conservative (Tukey) and powerful (t-test) tests of significance. Where significance was attained using both tests, only the Tukey test was reported. In each analysis, the homogeneity of variances was assessed using Bartlett's test for homogeneity, and for variables with non-homogenous variances, mean differences were measured using non-parametric analyses (Mann-Whitney U test for between-subjects designs; Friedman's and Wilcoxon tests for RM designs). All probabilities were two-sided.
Two patients randomized to the paroxetine group were removed from the study prior to initiating study medication or IFN-α therapy: one patient was discovered to have metastases and the other could not arrange consistent transportation. The data obtained for these two patients were not included in the dimensional analyses.
To resolve the issue of missing data due to exit of patients from the study prior to week 12, data were analyzed using the last observation carried forward method. Seven patients in the placebo group and one patient in the paroxetine group discontinued the study prior to week 12 due to severe depression or neurotoxicity. Other reasons for prematurely exiting the study included non-compliance with the study protocol (n = 1 in the paroxetine group) and the development of metastatic lesions or other medical complications requiring IFN-α discontinuation (n = 2 in the paroxetine group, n = 1 in the placebo group).
RESULTS
Socio-demographic Characteristics of the Study Sample
Socio-demographic characteristics of the study groups are depicted in Table 1 . There were no significant differences in the age (T = −0.57, df = 36, p = .58) or the ratio of males to females (χ2 = 0.12, df = 1, p = .73) between placebo- and paroxetine-treated patients. In addition, there were no differences between groups in education level (χ2 = 2.79, df = 3, p = .43), relationship status (χ2 = 1.81, df = 2, p = .41), or history of major depressive disorder (χ2 = 0.04, df = 1, p = .84).
IFN-α–induced Neurotoxicity in Placebo- and Paroxetine-treated Patients: Time Course and Symptom Severity
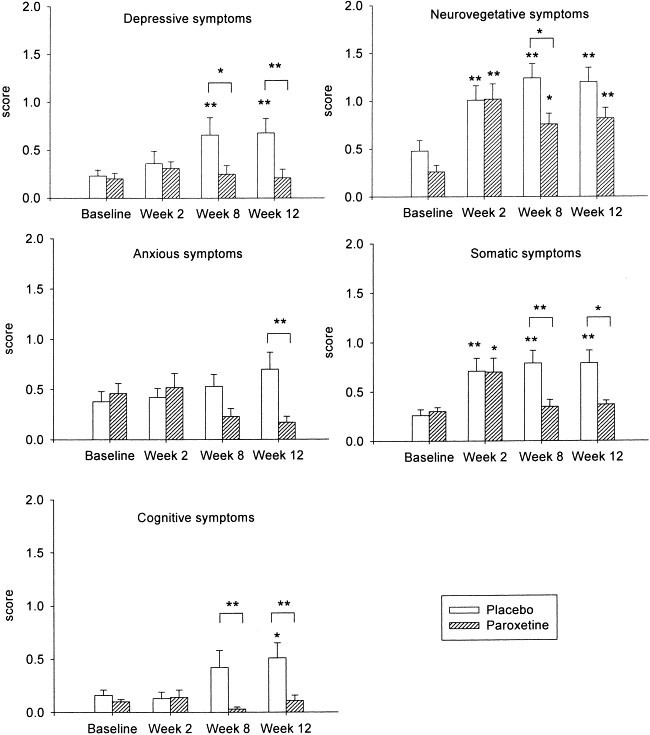
Baseline scores for neuropsychiatric and neurovegetative/somatic symptoms were similar in the two study groups ( Figure 1 and Table 2).
Depressive, anxious, cognitive, neurovegetative and somatic symptoms in placebo (n = 20) and paroxetine (n = 18) treated patients during IFN-α therapy.
Depressive symptoms were measured by the items of depressed mood, feelings of guilt and suicidal thoughts assessed in the HAM-D scale and anhedonia (loss of interest in people, loss of interest in work and activities) assessed in the NRS scale. Anxious symptoms included the symptoms of anxious mood, tension/irritability and fear assessed by the HAM-A scale. Cognitive symptoms were measured by the items of memory disturbances, distractibility, indecisiveness, word-finding problems and episodes of confusion from the NRS scale. Neurovegetative and somatic dimensions included symptoms of abnormal appetite, abnormal sleep (difficulty getting to sleep, difficulty staying asleep, sleeping too much), fatigue, psychomotor retardation, pain (headaches, body aches, joint pain) and gastrointestinal symptoms (nausea, vomiting, bowel problems) from the NRS scale.
Data are shown as mean + SEM. *:p < .05, **:p < .01. Asterisk at the top of bar indicates significant within-group difference vs. baseline. Brackets indicate inter-group difference for that time point.
Figure 1 depicts the temporal evolution of scores in each symptom dimension in placebo- versus paroxetine-treated patients over the course of the study. By week 12, placebo-treated patients displayed significantly higher scores, i.e., greater symptom severity, in the dimensions of mood, cognition, and neurovegetative and somatic symptoms compared with baseline. Depressive symptoms in placebo-treated patients were significantly increased above baseline at week 8 and remained elevated at week 12. Cognitive impairment in this group was significantly increased at week 12 compared with baseline. In contrast, neurovegetative and somatic symptoms in placebo-treated patients were significantly increased by the end of the second week of IFN-α treatment and remained elevated until the end of the study.
Paroxetine-treated patients displayed significantly higher scores during IFN-α therapy only in the dimensions assessing neurovegetative and somatic symptoms compared with baseline. As observed in placebo-treated patients, neurovegetative and somatic symptoms were present at the end of the second week of IFN-α therapy. Neurovegetative symptoms remained prominent during the duration of the study compared with baseline, whereas somatic symptoms were resolved by the end of the second month of IFN-α therapy in paroxetine-treated patients. At week 8, paroxetine-treated patients displayed significantly lower severity scores than placebo patients in the dimensions of depression, cognition, and neurovegetative and somatic symptoms. These differences in scores between placebo- vs. paroxetine-treated patients were also apparent at week 12, though, differences in neurovegetative symptoms between the groups did not attain statistical significance. Paroxetine-treated patients also displayed significantly lower scores than placebo patients in symptoms of anxiety at week 12.
As illustrated in Table 2, individual symptoms (within each symptom dimension) that increased in placebo-treated patients during IFN-α therapy included depressed mood, anhedonia, suicidal thoughts, memory disturbances, distractibility, anorexia, fatigue, psychomotor retardation, pain (headaches, body aches, joint pains) and gastrointestinal distress (nausea, vomiting, bowel problems). Data for week 8 were similar in each group compared with data for week 12 (data not shown). In placebo-treated patients, anorexia, fatigue, pain and gastrointestinal symptoms were apparent after two weeks of IFN-α treatment and remained elevated until the end of the study without significant worsening. In contrast, mood and cognitive symptoms and psychomotor retardation were only apparent at week 12. Individual symptoms that increased in paroxetine-treated patients during IFN-α therapy included anorexia, fatigue and gastrointestinal symptoms. Anorexia and fatigue developed at week 2 and remained apparent until the end of the study. Gastrointestinal symptoms were also apparent at week 2, but resolved by the end of the study. At the end of the study, paroxetine-treated patients displayed significantly lower scores than placebo-treated patients in the items assessing depressed mood, anxious mood, memory disturbances, distractibility, psychomotor retardation and pain.
Symptom Frequency in Placebo-treated Patients
Table 3 depicts the frequency of symptoms of at least moderate intensity (2 or greater on the NRS) experienced by placebo-treated patients during the course of IFN-α therapy. Among all the symptoms listed, fatigue was the symptom most frequently experienced by patients undergoing IFN-α therapy (80%), followed respectively by depressed mood (60%), pain (55%), gastrointestinal symptoms and tension/irritability (50%). A moderate percentage of IFN-α–treated patients reported cognitive symptoms, notably loss of concentration, which was endorsed by 30% of patients. Suicidal thoughts and feelings of guilt were reported in only 10% of placebo-treated patients during IFN-α therapy.
Symptoms Associated with IFN-α–induced Depression in Placebo-treated Patients
As previously reported, 9/20 (45%) patients in the placebo group fulfilled DSM-IV criteria for major depression at the end of the study (Musselman et al. 2001). Compared with their baseline scores, at week 12, these patients displayed significantly higher scores not only in the dimensions assessing depressive symptom severity (especially depressed mood, anhedonia and suicidal thoughts) but also in those reflecting both cognitive dysfunction (especially memory disturbances and episodes of confusion) and neurovegetative symptoms (anorexia, fatigue, and psychomotor retardation) ( Table 4). Of note, patients who developed syndromal major depression during IFN-α therapy displayed higher scores in the item assessing depressed mood after two weeks of IFN-α therapy compared with baseline. In addition, after two weeks of IFN-α therapy, these patients displayed higher scores on the items measuring anorexia, fatigue and gastrointestinal symptoms. At the end of the study (i.e., week 12), gastrointestinal symptoms had returned to their baseline values. In contrast to patients who became depressed, placebo patients who did not satisfy criteria for major depression during IFN-α therapy displayed significantly higher scores only in the neurovegetative items evaluating anorexia and fatigue, and somatic items measuring pain and gastrointestinal distress at week 12 compared with baseline. In these patients, anorexia, fatigue and gastrointestinal symptoms were also apparent at week 2. At week 12, depressed patients displayed significantly higher scores than non-depressed patients in the dimension of mood (especially depressed mood, anhedonia, tension and anxious mood), cognitive function (memory disturbances, loss of concentration and indecisiveness) and neurovegetative symptoms (especially fatigue and psychomotor retardation). However, at week 12, there was no significant difference between the two placebo subgroups in the dimension of somatic symptoms or anorexia.
DISCUSSION
Four main findings arise from the present study. First, the results demonstrate that IFN-α therapy is associated with the development of a wide range of symptoms, attesting to the interaction of this cytokine with various brain systems regulating mood, cognition, and neurovegetative and somatic function. Second, the data indicate that neurovegetative and somatic symptoms develop non-specifically in a large proportion of IFN-α-treated patients, whereas mood and cognitive effects of this cytokine are more apparent in patients who develop major depression. Third, neurovegetative and somatic symptoms appear early during IFN-α therapy, whereas mood and cognitive symptoms develop later. Finally, pretreatment with paroxetine is effective in preventing IFN-α–induced mood and cognitive symptoms as well as pain; however, the neurovegetative symptoms of anorexia and fatigue are less treatment responsive.
The development of neurotoxic effects in patients undergoing cytokine therapy has been demonstrated in previous reports (Valentine et al. 1998; Capuron et al. 2000). Nevertheless, based on a longitudinal design and dimensional analyses, results of the present study reveal distinguishing characteristics of IFN-α–induced behavioral symptoms. Neurovegetative and somatic symptoms developed early during the course of IFN-α therapy and remained apparent at later stages of therapy. In contrast, mood symptoms, especially depressed mood, and cognitive dysfunction (e.g. memory disturbances and distractibility) were minimal in severity at the end of the second week of IFN-α treatment and were most apparent later in the treatment period (week 8–12). In addition, mood and cognitive symptoms developed primarily in patients who developed major depression during the course of IFN-α therapy, whereas neurovegetative and somatic symptoms increased in both depressed and non-depressed subjects. The dissociation in terms of the temporal course of mood/cognitive versus neurovegetative/somatic symptoms suggests distinct pathophysiological mechanisms by which IFN-α affects brain systems regulating mood and cognition, and neurovegetative and somatic function. Consistent with this hypothesis is the distinction noted between these symptom dimensions regarding their responsiveness to paroxetine. Paroxetine prevented not only the development of mood symptoms during IFN-α administration, but also the appearance of cognitive dysfunction. In contrast, paroxetine had less effect on the development of the neurovegetative symptoms of anorexia and fatigue. Somatic symptoms (gastrointestinal distress and pain) also did not respond to paroxetine during the earlier stage of IFN-α therapy but were responsive at the end of the study. Dimensional analysis revealed that the effect of paroxetine on somatic symptoms was especially apparent with headaches and myalgia. Of note, paroxetine has been previously shown to improve chronic daily headaches (Foster and Bafaloukos 1994). These therapeutic effects of paroxetine may be related to the effects of antidepressants, including serotonin reuptake inhibitors, on pain and nociception (Schreiber et al. 1996; Barkin and Fawcett 2000; Lynch 2001).
Consistent with the effects of IFN-α on humans, acute injections of cytokines or cytokine inducers (e.g., lipopolysaccharide, LPS) to laboratory animals have been associated with a large and complex set of behavioral alterations referred to as “sickness behavior” (Dantzer et al. 1998; Yirmiya 1996). These behavioral changes resemble those observed in major depression and include decreased food consumption, reduced locomotor activity, anhedonia (as determined by decreased preference/consumption of sweetened liquids and reduced responsiveness in intracranial self stimulation paradigms), listlessness, social isolation, decreased libido, altered sleep patterns, hyperalgesia and cognitive dysfunction. Of note, all of these symptoms in laboratory animals develop relatively rapidly (within hours to days) after cytokine administration, depending on the cytokine administered, the route of administration (e.g., subcutaneous, intraperitoneal or intracerebraoventricular) and the duration of treatment (Kent et al. 1996; Dantzer et al. 1998; Lacosta et al. 1999). In addition, the majority of these symptoms, including decreased food consumption and decreased locomotor activity, can be prevented by pretreatment with antidepressants, including tricyclic antidepressants, SSRIs, and atypical antidepressants such as tianeptine (Castanon et al. 2001; Yirmiya et al. 2001) (although SSRIs have been found to exert a somewhat more variable and in some cases more limited effect on sickness behavior (Shen et al. 1999; Yirmiya et al. 2001)). In contrast to these studies in laboratory animals, our data in patients receiving IFN-α reveals a more complex behavioral response to cytokine administration with distinct temporal onset of qualitatively different symptom complexes with differential response to antidepressant administration. Nevertheless, differences between laboratory animal studies and our results may relate to the dose of cytokine used, the length of cytokine administration, and/or the nervous system response to IFN-α versus proinflammatory cytokines (e.g. TNF-α, IL-1 and IL-6) or LPS, more commonly used in animal studies.
There are many pathways by which IFN-α may cause neuropsychiatric complications including the induction of proinflammatory cytokines (Kent et al. 1992; Taylor and Grossberg 1998; Licinio et al. 1998), depletion of tryptophan (Brown et al. 1991), activation of the hypothalamic-pituitary adrenal (HPA) axis, presumably through stimulation of corticotropin releasing factor (CRF) release (Gisslinger et al. 1993), and reduction in the availability of thyroid hormone (Schultz et al. 1989; Fentiman et al. 1985). Given the noted differences in the appearance and treatment responsiveness of certain IFN-α–induced symptoms, it is reasonable to speculate that different pathways may mediate different symptom complexes during IFN-α treatment. Thus, while mood and cognitive symptoms appear to be mediated by pathways responsive to paroxetine, anorexia and fatigue appear to be mediated by separate pathway(s) that may respond to alternative interventions such as administration of cytokine receptor antagonists. Interestingly, similar to the findings reported here, the fatigue of patients diagnosed with chronic fatigue syndrome has been reported to be unresponsive to antidepressant treatment (Vercoulen et al. 1996). Other symptoms associated with chronic fatigue syndrome, including depression, insomnia and pain, appear to be more sensitive to antidepressant treatment (Wearden et al. 1998; Goodnick and Jorge 1999). In a related fashion, a recent study by Morrow et al. (2001) found that paroxetine significantly reduced depression but had no effect on fatigue in a double-blind randomized study of 738 cancer patients undergoing chemotherapy. In the present study, fatigue was the symptom most frequently reported by the placebo-treated patients undergoing IFN-α therapy, and like anorexia was relatively unresponsive to paroxetine treatment. Interestingly, fatigue and anorexia are common complications of a number of medical disorders. In fact, the symptoms of both fatigue and anorexia have been excluded from the diagnostic criteria for depression in the medically ill by some physicians, indicating their recognition that these symptoms reflect a “medical” pathology distinct from the pathophysiology of depression (Koenig et al. 1997). Of note, the existence of a fatigue syndrome separate from depressive symptoms has been documented in patients suffering from glandular fever and upper respiratory tract infections (White et al. 1995).
The mechanism(s) whereby paroxetine reduces IFN-α–induced mood and cognitive symptoms, psychomotor retardation and pain remain(s) to be determined. One possibility is that antidepressants like paroxetine may facilitate endogenous feedback pathways that regulate proinflammatory cytokines. The effects of TNF-α, IL-1 and IL-6 are downregulated by endogenous glucocorticoids (Auphan et al. 1995), and antidepressants have been shown to facilitate inhibitory feedback on cytokine secretion by endogenous glucocorticoids (Pariante and Miller 2001; Holsboer and Barden 1996). In addition, studies have shown that antidepressants may directly inhibit the release of proinflammatory cytokines (Maes et al. 1999). Antidepressants like paroxetine also enhance central nervous system serotonergic activity and thereby may counteract IFN-α–induced depletion of serotonin precursors (i.e. tryptophan) (Brown et al. 1991; Chaput et al. 1991). Finally, several antidepressants including paroxetine have been shown to reduce hypothalamic CRF mRNA expression and CRF biosynthesis (Holsboer and Barden 1996).
In summary, dimensional analyses reveal differential effects of IFN-α on mood, cognition and neurovegetative/somatic function in terms of symptom phenomenology, time course, and responsiveness to paroxetine. These differential effects suggest that different psychobiological mechanisms are responsible for the appearance of qualitatively distinct manifestations of mood and neurovegetative dysfunction following cytokine administration.
References
American Psychiatric Association. (1994): Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Washington, DC, American Psychiatric Association.
Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M . (1995): Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science 270: 286–290
Barkin RL, Fawcett J . (2000): The management challenges of chronic pain: the role of antidepressants. Am J Ther 7: 31–47
Brown RR, Ozaki Y, Datta SP, Borden EC, Sondel PM, Malone DG . (1991): Implications of interferon-induced tryptophan catabolism in cancer, auto-immune diseases and AIDS. Adv Exp Med Biol 294: 425–435
Capuron L, Ravaud A, Dantzer R . (2000): Early depressive symptoms in cancer patients receiving interleukin 2 and/or interferon alfa-2b therapy. J Clin Oncol 18: 2143–2151
Carpiniello B, Orru MG, Baita A, Pariante CM, Farci G . (1998): Mania induced by withdrawal of treatment with interferon alfa. Arch Gen Psychiatry 55: 88–89
Castanon N, Bluthe RM, Dantzer R . (2001): Chronic treatment with the atypical antidepressant tianeptine attenuates sickness behavior induced by peripheral but not central lipopolysaccharide and interleukin-1beta in the rat. Psychopharmacology 154: 50–60
Chaput Y, De Montigny C, Blier P . (1991): Presynaptic and postsynaptic modifications of the serotonin system by long-term administration of antidepressant treatments. An in vivo electrophysiologic study in the rat. Neuropsychopharmacology 5: 219–229
Dantzer R, Bluthe RM, Laye S, Bret-Dibat JL, Parnet P, Kelley KW . (1998): Cytokines and sickness behavior. Ann N Y Acad Sci 840: 586–590
Fentiman IS, Thomas BS, Balkwill FR, Rubens RD, Hayward JL . (1985): Primary hypothyroidism associated with interferon therapy of breast cancer. Lancet 1: 1166
Folstein MF, Folstein SE, McHugh PR . (1975): “Mini-mental State.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189–198
Foster CA, Bafaloukos J . (1994): Paroxetine in the treatment of chronic daily headache. Headache 34: 587–589
Gisslinger H, Svoboda T, Clodi M, Gilly B, Ludwig H, Havelec L, Luger A . (1993): Interferon-alpha stimulates the hypothalamic-pituitary-adrenal axis in vivo and in vitro. Neuroendocrinology 57: 489–495
Goodnick PJ, Jorge CM . (1999): Treatment of chronic fatigue syndrome with nefazodone. Am J Psychiatry 156: 797–798
Gutterman JU . (1994): Cytokine therapeutics: lessons from interferon alpha. Proc Natl Acad Sci USA 91: 1198–1205
Hamilton M . (1960): A rating scale for depression. J Neurol Neurosurg Psychiatry 23: 56–62
Holsboer F, Barden N . (1996): Antidepressants and hypothalamic-pituitary-adrenocortical regulation. Endocr Rev 17: 187–205
Kent S, Bluthe RM, Kelley KW, Dantzer R . (1992): Sickness behavior as a new target for drug development. Trends Pharmacol Sci 13: 24–28
Kent S, Bret-Dibat JL, Kelley KW, Dantzer R . (1996): Mechanisms of sickness-induced decreases in food-motivated behavior. Neurosci Biobehav Rev 20: 171–175
Kirkwood JM, Strawderman MH, Ernstoff MS, Smith TJ, Borden EC, Blum RH . (1996): Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol 14: 7–17
Koenig HG, George LK, Peterson BL, Pieper CF . (1997): Depression in medically ill hospitalized older adults: prevalence, characteristics, and course of symptoms according to six diagnostic schemes. Am J Psychiatry 154: 1376–1383
Lacosta S, Merali Z, Anisman H . (1999): Influence of acute and repeated interleukin-2 administration on spatial learning, locomotor activity, exploratory behaviors, and anxiety. Behav Neurosci 113: 1030–1041
Liang TJ, Rehermann B, Seeff LB, Hoofnagle JH . (2000): Pathogenesis, natural history, treatment, and prevention of hepatitis C. Ann Intern Med 132: 296–305
Licinio J, Kling MA, Hauser P . (1998): Cytokines and brain function: relevance to interferon-alpha-induced mood and cognitive changes. Semin Oncol 25 (suppl 1): 30–38
Lynch ME . (2001): Antidepressants as analgesics: a review of randomized controlled trials. J Psychiatry Neurosci 26: 30–36
Maes M, Song C, Lin AH, Bonaccorso S, Kenis G, De Jongh R, Bosmans E, Scharpe S . (1999): Negative immunoregulatory effects of antidepressants: inhibition of interferon-gamma and stimulation of interleukin-10 secretion. Neuropsychopharmacology 20: 370–379
Maier W, Buller R, Philipp M, Heuser I . (1988): The Hamilton Anxiety Scale: reliability, validity and sensitivity to change in anxiety and depressive disorders. J Affect Disord 14: 61–68
Meyers CA, Scheibel RS, Forman AD . (1991): Persistent neurotoxicity of systematically administered interferon-alpha. Neurology 41: 672–676
Miyaoka H, Otsubo T, Kamijima K, Ishii M, Onuki M, Mitamura K . (1999): Depression from interferon therapy in patients with hepatitis C. Am J Psychiatry 156: 1120
Morrow GR, Hickok JT, Raubertas RF, Flynn PJ, Hynes HE, Banerjee TK, Kirshner JJ, King DK . (2001): Effect of an SSRI antidepressant on fatigue and depression in seven hundred thirty-eight cancer patients treated with chemotherapy: a URCC CCOP study. Proc Am Soc Clin Oncol 20 (Abstr. 1531): 348a
Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS, Greiner K, Nemeroff CB, Miller AH . (2001): Paroxetine for the prevention of depression induced by high-dose interferon alfa. N Engl J Med 344: 961–966
Pariante CM, Miller AH . (2001): Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry 49: 391–404
Ravaud A, Bedane C, Geoffrois L, Lesimple T, Delaunay M . (1999): Toxicity and feasibility of adjuvant high-dose interferon alpha-2b in patients with melanoma in clinical oncologic practice. Br J Cancer 80: 1767–1769
Renault PF, Hoofnagle JH, Park Y, Mullen KD, Peters M, Jones DB, Rustgi V, Jones EA . (1987): Psychiatric complications of long-term interferon alfa therapy. Arch Intern Med 147: 1577–1580
Schreiber S, Backer MM, Yanai J, Pick CG . (1996): The antinociceptive effect of fluvoxamine. Eur Neuropsychopharmacol 6: 281–284
Schultz M, Muller R, Von zur Muhlen A, Brabant G . (1989): Induction of hyperthyroidism by interferon-alpha-2b. Lancet 1: 1452
Shen Y, Connor TJ, Nolan Y, Kelly JP, Leonard BE . (1999): Differential effect of chronic antidepressant treatments on lipopolysaccharide-induced depressive-like behavioural symptoms in the rat. Life Sci 65: 1773–1786
Taylor JL, Grossberg SE . (1998): The effects of interferon-alpha on the production and action of other cytokines. Semin Oncol 25(suppl 1):23–29
Valentine AD, Meyers CA, Talpaz M . (1995): Treatment of neurotoxic side effects of interferon-alpha with naltrexone. Cancer Invest 13: 561–566
Valentine AD, Meyers CA, Kling MA, Richelson E, Hauser P . (1998): Mood and cognitive side effects of interferon-alpha therapy. Semin Oncol 25(suppl 1):39–47
Vercoulen JH, Swanink CM, Zitman FG, Vreden SG, Hoofs MP, Fennis JF, Galama JM, Van der Meer JW, Bleijenberg G . (1996): Randomised, double-blind, placebo-controlled study of fluoxetine in chronic fatigue syndrome. Lancet 347: 858–861
Wearden AJ, Morriss RK, Mullis R, Strickland PL, Pearson DJ, Appleby L, Campbell IT, Morris JA . (1998): Randomised, double-blind, placebo-controlled treatment trial of fluoxetine and graded exercise for chronic fatigue syndrome. Br J Psychiatry 172: 485–490
White PD, Grover SA, Kangro HO, Thomas JM, Amess J, Clare AW . (1995): The validity and reliability of the fatigue syndrome that follows glandular fever. Psychol Med 25: 917–924
Yirmiya R . (1996): Endotoxin produces a depressive-like episode in rats. Brain Res 711: 163–174
Yirmiya R, Pollak Y, Barak O, Avitsur R, Ovadia H, Bette M, Weihe E, Weidenfeld J . (2001): Effects of antidepressant drugs on the behavioral and physiological responses to lipopolysaccharide (LPS) in rodents. Neuropsychopharmacology 24: 531–544
Acknowledgements
Supported by grants from the National Institute of Mental Health (MH00680 and MH60723), the Centers for Disease Control, Schering-Plough Pharmaceuticals, and Glaxo-SmithKline.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Capuron, L., Gumnick, J., Musselman, D. et al. Neurobehavioral Effects of Interferon-α in Cancer Patients: Phenomenology and Paroxetine Responsiveness of Symptom Dimensions. Neuropsychopharmacol 26, 643–652 (2002). https://doi.org/10.1016/S0893-133X(01)00407-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1016/S0893-133X(01)00407-9
Keywords
This article is cited by
-
The role of polyunsaturated fatty acids in the neurobiology of major depressive disorder and suicide risk
Molecular Psychiatry (2023)
-
Developing symptom clusters: linking inflammatory biomarkers to depressive symptom profiles
Translational Psychiatry (2022)
-
Depression treatment response to ketamine: sex-specific role of interleukin-8, but not other inflammatory markers
Translational Psychiatry (2021)
-
Interferon and anti-TNF therapies differentially modulate amygdala reactivity which predicts associated bidirectional changes in depressive symptoms
Molecular Psychiatry (2021)
-
Toll-like receptor 4-mediated cytokine synthesis and post-stroke depressive symptoms
Translational Psychiatry (2021)