Abstract
The effect of endogenous dopamine (DA) on measurement of neostriatal DA D2 receptor binding potential (D2RBP) in vivo was evaluated with positron emission tomography (PET) and the radiotracer [11C]raclopride by comparing the D2RBP before and after acute DA depletion. DA depletion was achieved by per-oral administration of 4.5 g α-methyl-para-tyrosine (AMPT) given in 25 h. Six healthy subjects completed the protocol. The AMPT treatment increased D2RBP significantly from 3.11 ± 0.25 to 3.68 ± 0.23 and decreased plasma levels of the DA metabolite homovanillic acid by 71 ± 11% and levels of the norepinephrine metabolite 3-methoxy-4-hydroxyphenethyleneglycol by 53 ± 7%. Increase in D2RBP correlated with decrease in attentiveness and with increase in errors of commission from Conners' Continuous Performance Test. On AMPT, a significant decrease in subjective happiness scores was observed. The results imply that a noninvasive [11C]raclopride PET protocol coupled with relatively brief administration of a rather low total dose of AMPT resulted in measurable acute DA depletion that might provide estimates of synaptic neostriatal DA concentration.
Similar content being viewed by others
Main
Endogenous dopamine (DA) levels have recently been estimated in humans in vivo with positron emission tomography (PET) and with single photon emission computed tomography (SPECT). DA levels during stimulant-induced release have been estimated by comparing radiotracer binding at baseline and after amphetamine or methylphenidate challenges using PET and the DA D2 receptor (D2R) radiotracer [11C]raclopride (Volkow et al. 1994; Breier et al. 1997) as well as SPECT and [123I](S)-(-)-3-iodo-2-hydroxy-6-methoxy-N-[(1-ethyl-2-pyrrolidinyl)methyl]benzamide ([123I]IBZM) (Booij et al. 1997; Abi-Dargham et al. 1998). DA levels during baseline release have been estimated by comparing radiotracer binding at baseline and after a rapid DA depletion induced by the competitive and reversible tyrosine hydroxylase inhibitor α-methyl-para-tyrosine (AMPT) (Engelman et al. 1968) using SPECT and [123I]IBZM (Laruelle et al. 1997; Abi-Dargham et al. 2000) or [123I]epidepride (Fujita et al. 2000).
One recent [123I]IBZM SPECT study showed a larger increase in neostriatal D2R availability with AMPT-induced DA depletion in 18 untreated schizophrenic patients compared to 18 matched controls (Abi-Dargham et al. 2000). These findings, which suggest that schizophrenic patients have elevated neostriatal DA levels, will need to be replicated in independent studies. In order to be able to do this with PET, we implemented a modified protocol for AMPT-induced DA depletion using the simplified 3-parameter reference tissue model (SRTM) (Lammertsma and Hume 1996) which only requires 60 min dynamic PET scanning following a bolus injection of [11C]raclopride. The good response of [11C]raclopride to changes in extracellular DA makes this tracer, so far, a good candidate for the PET measurement of changes in synaptic DA levels (Moresco et al. 1999; Laruelle 2000). This PET study design required more work before injection of the tracer and more effort analyzing the results after the data were acquired, but was very patient friendly, in contrast to the previously applied bolus infusion technique that required less work before and after imaging data acquisition, but that was not so patient friendly (Laruelle et al. 1997; Abi-Dargham et al. 2000).
In the two previous DA depletion imaging studies in healthy subjects, 8 g AMPT was administered over two days (Laruelle et al. 1997; Abi-Dargham et al. 2000), and 5.5 g/70 kg body weight of AMPT were given over 37 hours in a third study (Fujita et al. 2000). In the 67 healthy subjects who participated in these three studies, the following adverse effects were attributed to AMPT: eight developed acute dystonias, six had akathisia of sufficient severity to warrant psychotropic medication, four developed crystalluria, two showed significant increases in dysphoric mood states (anxiety and tension), and one diarrhoea. The administration of AMPT orally 1 g, t.i.d. for 24 hours has been reported to result in minimum levels of the DA metabolite homovanillic acid (HVA) and of the norepinephrine (NE) metabolite 3-methoxy-4-hydroxyphenethyleneglycol (MHPG) (Anand et al. 1999). HVA depletion following AMPT has been reported to reflect neostriatal DA depletion (Mignot and Laude 1985; Laruelle et al. 1997). Therefore, in order to prevent serious adverse effects of AMPT but still obtain adequate DA depletion, we decided to reduce the total amount of AMPT administered to 4.5 g orally over 28 hours. AMPT plasma levels were measured to assess whether this dosing regimen resulted in a sufficient AMPT concentration to substantially inhibit tyrosine hydroxylase (Laruelle et al. 1997). The amount of catecholamine depletion was also estimated by measuring plasma levels of HVA, MHPG and prolactin.
Previously AMPT has been reported to induce not only acute dystonias in a minority but also mild signs of Parkinsonism in the majority of healthy subjects (Laruelle et al. 1997; Abi-Dargham et al. 2000; Fujita et al. 2000). We, therefore, monitored our subjects regularly for possible extrapyramidal symptoms. Since AMPT was reported to affect subjective feelings in healthy subjects with significant decreases in happiness and increases in sleepiness and restlessness (McCann et al. 1993; Laruelle et al. 1997), subjects reported subjective feelings both in a continuous visual analog and in an ordinal scale fashion.
As alterations in DAergic transmission may be involved in selective attention (Servan-Schreiber et al. 1998), this was tested in the healthy subjects at baseline and at different stages of AMPT-induced DA depletion. A finger tapping task was included in order to control for possible effects of DA depletion on motor speed. Since self-reported sleepiness during AMPT depletion has been reported to be a good predictor of poor performance on cognitive tests (McCann et al. 1992), objective ratings were obtained for sedation during each series of cognitive tests.
MATERIALS AND METHODS
Human Subjects
The study was approved by the Human Subjects Review Committee of the University of Toronto and has been carried out in accordance with the Helsinki Declaration of 1975. Five men and three women, age 27 ± 5 years (all values in this article are expressed as average ± standard deviation) and all right-handed, entered the study. Exclusion criteria were: 1) psychiatric diagnosis on Axis I as assessed by the Structured Clinical Interview for DSM-IV, nonpatient version; 2) serious medical or neurological illness or significant head injury by history or on physical examination or based on laboratory studies (complete blood count, fasting blood glucose, basic urea nitrogen, creatinine, electrolytes, thyroid function tests, liver function tests, urinalysis, EKG); 3) lifetime history of alcohol or substance dependence; 4) history of alcohol or substance abuse during the six months preceding the study; 5) recent substance use on urine toxicology screen; 6) treatment with any psychotropic medications by history or on urine toxicology screen; and 7) pregnancy by history or on urine βHCG test.
Depletion Regimen and Clinical Monitoring
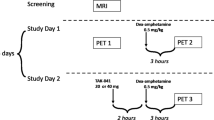
Each subject was scanned twice, in the baseline state (PET1, day 1) and after DA depletion (PET2, day 3). DA depletion was induced by oral administration of in total 4500 mg AMPT over 25 hours. AMPT was administered orally in doses of 750 mg each at the following times: at 10AM, 1.30PM, 6PM, and 10PM on day 2, and at 7AM and 11AM on day 3. During AMPT administration, subjects remained under direct observation at the PET Centre during the day and on a psychiatric inpatient unit during the night. To prevent the formation of AMPT crystals in the urine, subjects were instructed to drink at least 4 L of fluids per day, starting on day 2 (Engelman et al. 1968). In addition, in order to alkalinize the urine which increased AMPT solubility, sodium bicarbonate 1.2 g orally was given at 10 p.m. on day 1 and at 7 a.m. on day 2. Urine samples were collected at 3 p.m. on day 2 and at 7 a.m. on day 3 to examine the presence of AMPT crystals.
Subjects were evaluated five times using clinical rating scales for adverse effects and mood states pre AMPT (on day 1 and on day 2) and post AMPT (cumulative oral doses of 750 mg on day 2, and 3750 mg and 4500 mg on day 3). The presence of adverse effects such as parkinsonian symptoms, acute dystonias, and abnormal involuntary movements, was monitored using the Extrapyramidal Symptom Rating Scale (ESRS) (Chouinard et al. 1980). The subjects rated 19 subjective feelings on a continuous visual analog scale (VAS) ranging from 0% (“not at all”) to 100% (“most ever”). Subjective feelings were also rated using the ordinal Profile Of Mood States (POMS) (McNair et al. 1981). In addition, subjects rated depressive symptoms using the Beck Depression Inventory, Short Form (BDI) (Beck et al. 1974).
The subjects performed the Conners' Continuous Performance Test (CPT) (Conners 1995) and the Finger Tapping Test (FTT) (Reitan and Wolfson 1985) pre AMPT (day 1), post 1500 mg AMPT (day 2), and post 3750 mg AMPT (day 3). The FTT was applied using a Finger Tapper board (Psychological Assessment Resources, Inc., Odessa, FL). After one 10-second practice trial for each hand, 10-second trials were administered alternating between the right and left hand, allowing for a 15-second rest period between trials for each hand. Trials were administered until five consecutive trials rendered scores within a five-tap range, and average scores of these five trials were obtained. If this criterion was not reached, 10 trials were administered and average scores of these 10 trials were obtained. Subjects were rated during these tests regarding level of sedation using the Observer's Assessment of Alertness/Sedation Scale (OAASS) (Chernik et al. 1990).
Catecholamine Metabolites Plasma Analysis
Plasma HVA and MHPG samples were collected at 10 a.m. (day 1), at 10 a.m. (day 2 pre AMPT), at 3 p.m. (day 2 post 1500 mg AMPT), and at 1 p.m. (day 3 post 4500 mg AMPT).
Plasma HVA levels were measured as the methylated then acetylated derivative using Gas Chromatography-Mass spectrometry (GCMS) with selected ion monitoring (Warsh et al. 1987). Plasma MHPG levels were measured as the 4-acetyl-di-trifluoro-acetyl derivative using GCMS with selected ion monitoring (Takahashi et al. 1977).
AMPT Plasma Analysis
Plasma AMPT samples were collected at 10 a.m. (day 1), at 10 a.m. (day 2 pre AMPT), at 3 p.m. (day 2 post 1500 mg AMPT), and at 1 p.m. (day 3 post 4500 mg AMPT). Plasma AMPT concentrations were measured as the pentafluorobenzoyl derivative using GCMS with selected ion monitoring (Roy et al. 1984).
Prolactin Plasma Analysis
Plasma prolactin samples were collected at 10 a.m. (day 1), at 10 a.m. (day 2 pre AMPT), at 3 p.m. (day 2 post 1500 mg AMPT), and at 1 p.m. (day 3 post 4500 mg AMPT). Plasma prolactin levels were measured using microparticle enzyme immunoassay technology (Abbott Laboratories 1997).
[11C]raclopride PET Data Acquisition
PET images were obtained with a GEMS PC2048–15B camera (General Electric Medical Systems, Milwaukee, WI) in five 1-minute frames followed by twenty 2-minute frames and three 5-minute frames after [11C]raclopride bolus injection (pre AMPT: 370 ± 34 MBq (9.99 ± 0.93 mCi), specific activity 53,095 ± 17,649 GBq/mmol (1435 ± 477 Ci/mmol); post AMPT: 385 ± 42 MBq (10.41 ± 1.14 mCi), specific activity 56,795 ± 18,648 GBq/mmol (1535 ± 504 Ci/mmol)). There was no significant difference in the pre- and post-AMPT injected radioactivity and specific activity (two-tailed paired Student's t-test: df = 5, p = .552 and p = .673, respectively). The images were corrected for attenuation with a 68Ge transmission scan and reconstructed using filtered back projection (Hanning filter, 5 mm full width at half maximum) and fifteen 6.5 mm-thick transaxial slices were obtained (total axial field of view 9.75 cm; covering area from the canto-meatal line upwards; part of cerebral cortex from vertex downward not covered).
Image Analysis
Regions of interest (ROIs) were manually drawn following the contour of the striata (pre AMPT: 3371 ± 465 mm3; post AMPT: 3202 ± 553 mm3) and cerebellum (pre AMPT: 13893 ± 1549 mm3; post AMPT: 13698 ± 1264 mm3) on two adjacent transaxial PET slices. There were no significant differences in the pre- and post-AMPT sizes of the ROIs of the striata and cerebellum (two-tailed paired Student's t-test: df = 5, p = .394 and p = .819, respectively). For [11C]raclopride PET data, ROIs selected from the PET images have been shown to be almost identical to those obtained from coregistered MRI images (Wang et al. 1996a).
The neostriatal DA D2 receptor binding potential (D2RBP), the product of the total D2R density (Bmax), and the affinity (1/Kd) of [11C]raclopride for D2R, were calculated using the SRTM (Lammertsma and Hume 1996). For [11C]raclopride PET data, the SRTM has been shown to provide similar results as the 4-parameter reference tissue model (Lammertsma and Hume 1996), whereas the 4-parameter reference tissue model produced similar results as 1- and 2-tissue compartment models requiring metabolite-corrected plasma curves (Lammertsma et al. 1996; Ito et al. 1998) and as the sustained equilibrium method after bolus plus continuous infusion (Ito et al. 1998). The SRTM was applied using a flexible kinetic modeling tool (Burger and Buck 1997) which also allowed us to construct parametric D2RBP images (Gunn et al. 1997). The parametric D2RBP images were spatially normalized within the standard Montreal Neurologic Institute brain space using Statistical Parametric Mapping version 99 (SPM99) (Friston et al. 1995) and the D2R ligand specific template technique (Meyer et al. 1999).
Outcome Measures
Our method is based on the finding that endogenous DA competes with the binding of [11C]raclopride. When tracer amounts of high-specific activity [11C]raclopride are injected and approach equilibrium, the ratio of the [11C]raclopride bound specifically to the D2R (Srac) to that which is free in the extracellular fluid close to the D2R (Frac) is equal to the product of Bmax times 1/Kd, provided there is no endogenous DA: 
As Frac cannot be measured directly, this is estimated from the count rate density (i.e., Frac plus nonspecific binding) in the cerebellum, a brain region almost devoid of D2R (Martres et al. 1985). However, in vivo the endogenous DA competes with [11C]raclopride for the D2R. This competition can be represented as:  where D2RBPbaseline = D2RBP measurement at baseline, DAconc = neostriatal DA concentration, and Ki = equilibrium inhibitory constant for DA regarding inhibition of raclopride binding.
where D2RBPbaseline = D2RBP measurement at baseline, DAconc = neostriatal DA concentration, and Ki = equilibrium inhibitory constant for DA regarding inhibition of raclopride binding.
However, D2RBPbaseline is confounded by endogenous DA. If one depletes DA and thereby removes the competition and the DAconc term in the denominator, one can obtain a more accurate estimate of D2RBP (D2RBPdepleted). Therefore, under DA depletion, equation 2 functionally becomes: 
The foregoing discussion illustrates that: 1) D2RBPbaseline is confounded by endogenous DA, and the higher the concentration of DA the lower the value of D2BP that will be obtained; 2) D2RBPdepleted more accurately reflects the true status of D2R; 3) by combining equations 2 and 3 it can be shown that the fractional increase in D2RBP [i.e., (D2RBPdepleted − D2RBPbaseline)/D2RBPbaseline = D2RBPshift] is linearly proportional to the baseline DAconc, provided the process of DA depletion does not change the number and affinity of the D2R. Thus, D2RBPshift, under appropriate assumptions, is a semiquantitative index of endogenous DA levels.
Statistical Analyses
Data were monitored for meeting the criteria for a normal distribution by testing for skewness, curtosis and outlyers, and for homogeneity of variance (Tabachnick and Fidell 1996). Correlations between PET data and clinical parameters were tested for using Pearson's product-moment correlation coefficient (r) if the criteria for a normal distribution were met and using Spearman's rank correlation coefficient (Rho) if these criteria were not met. AMPT effects on clinical ratings and plasma levels were assessed by repeated measures ANOVA, if the criteria for a normal distribution and homogeneity of variance were met and by Friedman's test if these criteria were not met. Similarly, AMPT effects on PET measurements were assessed by two-tailed paired Student's t-tests if the criteria were met and by Wilcoxon's signed ranks test if the criteria were not met. All tests were 2-tailed and probability values of 0.05 were used as the significance level. No corrections for multiple comparisons were applied. Statistical analyses were performed with SPSS for Windows, release 10.0.0 (SPSS Inc., Chicago, IL, 1999).
For the parametric D2RBP images the equivalent of a 2-tailed paired Student's t-test pre versus post AMPT was done using SPM99. This was done because: 1) increased uptake has been observed in similar extrastriatal regions with [11C]raclopride PET (Wang et al. 1993) as with [123I]epidepride SPECT (Fujita et al. 2000), suggesting visualization of extrastriatal D2R; 2) decrements in uptake in some of those regions (i.e., in the thalamus and temporal insula) have been reported with age, in concordance with the D2R decline with age in those regions observed post mortem (Wang et al. 1996b); and 3) baseline occupancy of extrastriatal D2R by dopamine has been demonstrated using [123I]epidepride SPECT and AMPT-induced dopamine depletion (Fujita et al. 2000).
As SPM has been reported to be successful in detecting receptor changes in brain areas that were not defined a priori (Weeks et al. 1997), this technique might help us in detecting possible increases in extrastriatal D2RBP post AMPT. No global normalization was carried out. This provided a comparison between groups of the D2RBP values. A threshold of 80%, discarding all values smaller than 80% of the whole brain average D2RBP value, was used to delineate gray matter voxels. Corrected p-values < .05 at cluster or voxel level were considered significant.
RESULTS
Compliance with Protocol
Four men and two women, age 27 ± 6 years (average ± s.d.) completed the protocol. A 30-year-old man and a 24-year-old woman initiated the study but did not complete the protocol. The man left due to mild symptoms (moderate dizziness when standing up, mild restlessness, mild hand tremors and mild slowness of movement) and the woman left the study as she did not like the overnight stay in the psychiatric inpatient unit.
[11C]raclopride PET
The D2RBP, obtained using manually drawn ROIs, was 3.11 ± 0.25 at baseline and increased significantly to 3.68 ± 0.23 post AMPT (paired t-test: t = −22.666, df = 5, p < .001) (Figure 1). The D2RBPshift was 0.185 ± 0.030.
Comparison of neostriatal dopamine D2 receptor binding potential (D2RBP), determined from Positron Emission Tomography data obtained from 0 to 60 min after [11C]raclopride bolus injection, in six healthy subjects pre versus post AMPT. In each subject, the D2RBP post AMPT was larger than pre AMPT (2-tailed paired t-test: t = −22.666, p < .001)
The analysis of the parametric D2RBP images in SPM99 showed two clusters of significant change at the levels of the right and left striatum, respectively (corrected p-values for both clusters < .0001) (Figure 2). No significant differences at cluster or voxel level were observed in any other brain regions.
Results from the Statistical Parametric Mapping 99 analysis of the parametric dopamine D2 receptor binding potential (D2RBP) images in six healthy subjects pre versus post AMPT determined from Positron Emission Tomography data obtained from 0 to 60 min after [11C]raclopride bolus injection. Transaxial slices of spatially normalized brains within the standard Montreal Neurologic Institute brain space in standard neurological orientation. The slices are parallel to and from 2 mm to 18 mm above the intercommissural line. Voxels with significant T statistics (corrected p-values < .05), indicating significant D2RBP increases post versus pre AMPT, are indicated in hot color scale (ranging from 0–20 for the T statistic values)
A negative correlation was observed between age and D2RBPbaseline (r = −0.941, p = .005). This correlation with age persisted for D2RBPdepleted (r = −0.833; p = .040) since D2RBPbaseline and D2RBPdepleted were positively correlated (r = 0.969, p = .001). There was a trend for a negative correlation between D2RBPshift and D2RBPbaseline (r = −0.788, p = .063).
Plasma Measurements
Plasma Catecholamine Metabolites
Effects of AMPT on plasma HVA and MHPG levels are shown in Table 1. The AMPT-induced decrease in plasma HVA was significantly larger than that in MHPG (paired t-test, df = 5, t = 4.409, p = .007).
Plasma AMPT
AMPT levels were 10 ± 3 μg/mL on day 2 post 1500 mg AMPT and 21 ± 12 μg/mL on day 3 post 4500 mg AMPT.
Plasma Prolactin
Effects of AMPT on plasma prolactin levels are shown in Table 1. AMPT significantly increased plasma prolactin.
Clinical Effects of AMPT
Only very mild Parkinsonian symptoms and akathisia were induced by AMPT in three of our subjects. No acute dystonias or abnormal involuntary movements were observed. Effects of AMPT on VAS scores are shown in Table 2. On AMPT, scores for happiness were significantly decreased whereas scores for tiredness, sleepiness and drowsiness were significantly increased. There was a tendency for subjects to feel less energetic on AMPT whereas scores for hungriness were quite variable at different times without a clear dose dependence on AMPT. No significant changes on AMPT were observed for the other VAS scores, in order of decreasing significance: talkative, depressed, anxious, high, nervous, sad, irritable, calm, mellow, fearful, mania, restless, and angry. Percentage decrease in happiness scores was significantly correlated to percentage MHPG decrease (r = 0.935, p = 0.006) but not to percentage HVA decrease or to D2RBPshift.
None of six dimensions derived from POMS scores (in order of decreasing significance: vigor, confusion, fatigue, tension, depression, and anger) changed significantly on AMPT. BDI scores were very low and did not change with AMPT treatment.
Effects of AMPT on cognitive test performance are shown in Table 3. The OAASS overall scores decreased significantly on AMPT. Significant effects of AMPT on several CPT items were observed: Number of Commissions and Hit Response Time Standard Error increased whereas Attentiveness (d') decreased. Other CPT items showed trends for significance on AMPT: Hit Response Time Change over Inter Stimulus Intervals and the Overall Index increased. The remaining CPT items did not show any (trend for) significant change on AMPT.
D2RBPshift was significantly correlated with percentage increase in Number of Commissions (r = 0.837, p = .038) and with percentage decrease in Attentiveness (d') (r = 0.890, p = .018). No significant AMPT-induced changes or correlations with age were noted on FTT scores (left and right index fingers).
DISCUSSION
[11C]raclopride PET
An 18.5 ± 3.0% neostriatal D2RBP increase was observed in the six subjects who completed the AMPT DA depletion protocol. This is in accordance with D2RBP increases reported with [123I]IBZM SPECT in two independent samples: 28 ± 16% in nine healthy subjects 25 ± 4 years old (Laruelle et al. 1997) and 9 ± 7% in 18 healthy subjects 31 ± 8 years old (Abi-Dargham et al. 2000).
Assuming partial depletion, we estimated a DAconc of 27 ± 6 nM [by multiplying the DAconc, calculated assuming full depletion and taking for Ki a value of 100 nM (Fisher et al. 1995; Ginovart et al. 1997), with HVAPET1/(HVAPET1-HVAPET2) (Laruelle et al. 1997)]. Estimations in vivo for the DAconc have been 40–60 nM in mice (Ross and Jackson 1989), 51–56 nM in Cynomolgus monkeys (Ginovart et al. 1997), 45 ± 25 nM (assuming full depletion) to 72 ± 40 nM (assuming partial depletion) in humans (Laruelle et al. 1997). Based on a literature review, the average DAconc in humans has been estimated to be 100 nM at baseline and 200 nM during activation (Fisher et al. 1995). Therefore, our estimation of the DAconc at baseline is in the same order of magnitude as estimations from the literature. However, caution should be exercised when estimating the baseline DAconc from the D2RBPshift, as we did in this study, since at least eight potential in vivo modulators of the D2RBPshift must be considered.
-
1
The AMPT dose used in our study may not have resulted in optimum DA depletion with minimal adverse effects. Our dose rate was similar to the 1 g PO q.i.d. administered by Laruelle et al. (1997) and Abi-Dargham et al. (2000), whereas the duration was similar to the 24 hours suggested by Anand et al. (1999). We performed clinical ratings and plasma metabolite measures at various points during our study to monitor the effect of various total dosages of AMPT. Validation of these preliminary results may require producing placebo-controlled dose response curves.
-
2
In our study, the order of PET scanning at baseline and with DA depletion was not randomized. This because of a possible carry-over effect if PET on AMPT depletion would have been done first. Thus, we cannot fully rule out a time or order effect on our imaging data.
-
3
The neostriatal D2RBP increase after AMPT administration might have reflected D2R upregulation rather than removal of endogenous DA. However, previous animal studies indicated that dopamine depletion for two days did not cause receptor upregulation. In rodents, DA depletion induced by reserpine (Ross and Jackson 1989), 6-hydroxydopamine (Iwata et al. 1992; Hume et al. 1995; Narang and Wamsley 1995) or AMPT (Laruelle et al. 1997) did not cause any significant neostriatal D2R upregulation in two days to three weeks. In Cynomolgus monkeys, reserpine-induced DA depletion did not change Bmax in vivo (Ginovart et al. 1997). Given the brief duration of our AMPT administration protocol, receptor upregulation does not seem to have contributed to the D2RBP increase.
-
4
Differences in neostriatal D2RBP increase could reflect different proportions of high- and low-affinity state D2R. In general, agonists predominantly label high-affinity state and antagonists label both high- and low-affinity state D2R (Sibley et al. 1982). The proportion of these two states varies among brain regions (Camps et al. 1989) and may differ in vivo from in vitro (Richfield et al. 1986).
-
5
It is generally believed that agonists cause internalization of D2R (Ito et al. 1999), which in vivo results in an increased affinity for butyrophenones and in a decreased affinity for benzamides (Laruelle 2000). The latter may be due to the changed internal milieu with D2R internalization such as decreasing sodium and increasing proton concentrations which are both known to decrease the affinity of benzamides for D2R (Laruelle 2000). It is unlikely to be due to decreased access of benzamides to internalized D2R since all effective neuroreceptor ligands must pass the blood brain barrier, which is essentially composed of a lipophilic membrane. Thus, such tracers should pass the plasma membrane and have access to internalized receptors. This is supported by data showing that [123I]IBZM diffuses well within peripheral blood cells in humans in vivo (Costa et al. 1990).
-
6
There may be interindividual differences in pharmacodynamic effects of AMPT on tyrosine hydroxylase. In fact, four isoforms of this enzyme were found in human brain showing different regional distributions (Lewis et al. 1993).
-
7
A significant number of D2R are located extrasynaptically (Khan et al. 1998) where the DA concentration is lower than in the synapse (Fisher et al. 1995). Thus, DA may occupy a smaller percentage of extrasynaptic receptors compared to those within the synapse and there may be interindividual differences in the proportion of synaptic versus extrasynaptic D2R.
-
8
Benzamides label both dimers and monomers of D2R whereas butyrophenones label only monomers (Verhoeff 1999; Laruelle 2000). However, the actual relationship between D2R oligomeric states, D2R internalization and vulnerability to endogenous DA competition has to our knowledge not been documented (Laruelle 2000).
We observed a negative correlation between age and neostriatal D2RBP at baseline which was preserved after DA depletion. Negative correlations between age and neostriatal D2RBP have been consistently reported in imaging studies of healthy subjects in which endogenous DA competed with the radiotracers used (Verhoeff 1999). The preliminary findings in our study suggest that these negative correlations may remain valid with DA depletion.
A trend for a negative correlation between D2RBPshift and D2RBPbaseline was observed both in our study and by Laruelle et al. (1997). This suggests that a relatively high D2R occupancy by DA might contribute to a relatively low D2RBPbaseline.
Plasma Catecholamine Metabolites
The AMPT-induced decreases in plasma HVA by 71 ± 11% and in plasma MHPG by 53 ± 7% in our study are compatible with the decreases of 70 ± 12% and 66 ± 6%, respectively, obtained by Laruelle et al. (1997) and with the decreases of 64 ± 11% and 51 ± 11%, respectively, observed by Fujita et al. (2000).
Plasma AMPT
The AMPT levels in our study were 21.1 ± 11.7 μg/mL prior to PET2. These values are compatible with the steady-state levels of 21 ± 7 μg/mL obtained by Laruelle et al. (1997) and 21.4 ± 8.0 μg/ml obtained by Fujita et al. (2000).
Plasma Prolactin
The AMPT-induced increases in plasma prolactin by 340 ± 249% on day 2 and by 176 ± 118% on day 3 were larger than the 93 ± 56% (rested) and 152 ± 86% (sleep-deprived) increases observed post 5250 mg AMPT over a 33-hour period in 40 healthy male volunteers (McCann et al. 1992). The increases in prolactin in our study were larger in the two women (range on day 2: 241–820%; on day 3: 204–390%) than in the four men (range on day 2: 100–352%; on day 3: 40–170%). An increased AMPT-induced prolactin secretion in women has also been observed by Zimmermann et al. (1996) and this gender difference could well be the reason for the larger prolactin increases in our study than those observed by McCann et al. (1992).
As prolactin levels have been reported to be constant between 10 a.m. and 5 p.m. (Sassin et al. 1972), the higher levels on day 2 than on day 3 cannot be ascribed to circadian variation. The decreasing prolactin levels with increasing duration on AMPT may be due to the fact that the rise in prolactin may be related to the rate of DA depletion rather than to a constant amount of DA depletion, and that with more constant DA depletion internal feedback circuits are increasingly able to mitigate the effect of DA depletion on prolactin levels.
Clinical Effects of AMPT
The significant decreases in happiness and increases in tiredness and sleepiness VAS scores on AMPT in our study have also been observed by others in healthy subjects (McCann et al. 1993; Laruelle et al. 1997). The fact that decrease in happiness was highly and significantly correlated to MHPG decrease but not HVA decrease suggests a larger role for NE depletion than for DA depletion.
Moreover, we could not confirm the significant positive correlation between D2RBP increase and decrease in VAS happiness scores described by Laruelle et al. (1997). This may be due to the fact that not DA but NE depletion seems to be more related to the decrease in happiness. However, interpretation of our results is limited by: 1) the lower number of subjects reducing the power to detect such correlations; and 2) the lack of a placebo group.
Attentiveness (d'), the measure for selective attentiveness of the CPT, decreased significantly on AMPT and this decrease was significantly positively correlated with D2RBPshift. In parallel, the number of errors of commission increased significantly on AMPT and this increase was significantly positively correlated with D2RBPshift. These data are in accordance with data showing an improvement in selective attention in healthy subjects after d-amphetamine administration in healthy subjects (Servan-Schreiber et al. 1998). Based on those data and on our study, it seems that increasing DAergic transmission improves and decreasing DAergic transmission worsens selective attention in healthy subjects. Whereas d-amphetamine induced a speeding of reaction time overall and an improvement of accuracy at fast reaction times (Servan-Schreiber et al. 1998), AMPT in our study did not affect reaction time and reduced accuracy by increasing errors of commission.
Since the FTT results in our study were unaffected by AMPT administration, AMPT was found to primarily affect catecholamine transmission in cognitive rather than motor networks. This was similar for d-amphetamine in the study of Servan-Schreiber et al. (1998). However, the lack of any significant change of FTT scores with AMPT-induced DA depletion and with age in our study is in contrast with findings of Volkow et al. (1998) who observed significant and high positive correlations between D2R availability and FTT scores, both without and with partialing out the significant age effect in their study on both D2R availability and FTT scores. Given the limited sample size and age range in this study, we do not feel confident that we can rule out an age effect on the FTT scores. The D2RBP decrease with age is more chronic and likely to be differentially related to synaptic DA levels and DA transmission than the acute D2RBP increase on AMPT, therefore resulting in a differential relationship with FTT scores. We intend to expand the sample size and age range of healthy subjects to obtain more informative data on these relationships.
We conclude that the noninvasive [11C]raclopride PET protocol used in our study, coupled with relatively brief administration of a relatively low total dose of AMPT, resulted in measurable acute DA depletion that might reflect estimates of synaptic neostriatal DA concentration.
References
Abbott Laboratories. (1997): Imx® Prolactin. Abbott Park, Il, Abbott Laboratories, pp 1–11
Abi-Dargham A, Gil R, Krystal J, Baldwin RM, Seibyl JP, Bowers M, van Dyck CH, Charney DS, Innis RB, Laruelle M . (1998): Increased striatal dopamine transmission in schizophrenia: Confirmation in a second cohort. Am J Psychiatry 155: 761–767
Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, Weiss R, Cooper TB, Mann JJ, VanHeertum RL, Gorman JM, Laruelle M . (2000): Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci U S A 97: 8104–8109
Anand A, Darnell A, Miller HL, Berman RM, Cappiello A, Oren DA, Woods SW, Charney DS . (1999): Effect of catecholamine depletion on lithium-induced long-term remission of bipolar disorder. Biol Psychiatry 45: 972–978
Beck AT, Rial WY, Rickels K . (1974): Short form of depression inventory cross-validation. Psychol Rep 34 (3) :1184–1186
Booij J, Korn P, Linszen DH, van Royen EA . (1997): Assessment of endogenous dopamine release by methylphenydate challenge using iodine-123 iodobenzamide single-photon emission tomography. Eur J Nucl Med 24: 674–677
Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A, Weinberger DR, Weisenfeld N, Malhotra AK, Eckelman WC, Pickar D . (1997): Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: Evidence from a novel positron emission tomography method. Proc Natl Acad Sci U S A 94: 2569–2574
Burger C, Buck A . (1997): Requirements and implementation of a flexible kinetic modeling tool. J Nucl Med 38: 1818–1823
Camps M, Cortés R, Gueye B, Probst A, Palacios JM . (1989): Dopamine receptors in human brain: autoradiographic distribution of D2 sites. Neuroscience 28: 275–290
Chernik DA, Gillings D, Laine H, Hendler J, Silver JM, Davidson AB, Schwam EM, Siegel JL . (1990): Validity and reliability of the Observer's Assessment of Alertness/Sedation Scale: Study with intravenous midazolam. J Clin Psychopharmacol 10: 244–251
Chouinard G, Ross-Chouinard A, Annable L, Jones BD . (1980): The extrapyramidal symptom rating scale. Can J Neurol Sci 7: 233
Conners CK . (1995): Conners' Continuous Performance Test computer program. User's manual. Toronto, Ontario, Canada, Multi-Health Systems, Inc.
Costa DC, Verhoeff NPLG, Cullum ID, Ell PJ, Syed GMS, Barrett J, Palazidou E, Toone B, van Royen EA, Bobeldijk M . (1990): In vivo characterisation of 3-iodo-6-methoxybenzamide 123I in humans. Eur J Nucl Med 16: 813–816
Engelman K, Jequier E, Udenfriend S, Sjoerdsma A . (1968): Metabolism of alpha-methyltyrosine in man: Relationship to its potency as an inhibitor of catecholamine biosynthesis. J Clin Invest 47: 568–576
Fisher RE, Morris ED, Alpert NM, Fischman AJ . (1995): In vivo imaging of neuromodulatory synaptic transmission using PET: A review of relevant neurophysiology. Hum Brain Mapping 3: 24–34
Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiack RSJ . (1995): Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapping 2: 189–210
Fujita M, Verhoeff NPLG, Varrone A, Zoghbi SS, Baldwin RM, Jatlow PA, Anderson GM, Seibyl JP, Innis RB . (2000): Imaging extrastriatal dopamine D2 receptor occupancy by endogenous dopamine in healthy humans. Eur J Pharmacol 387: 179–188
Ginovart N, Farde L, Halldin C, Swahn CG . (1997): Effect of reserpine-induced depletion of synaptic dopamine on [11C]raclopride binding to D2-dopamine receptors in the monkey brain. Synapse 25: 321–325
Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ . (1997): Parametric imaging of ligand-receptor binding in PET using a simplified reference tissue model. Neuroimage 6: 279–287
Hume SP, Opacka-Juffry J, Myers R, Ahier RG, Ashworth S, Brooks DJ, Lammertsma AA . (1995): Effect of L-dopa and 6-hydroxydopamine on [11C]raclopride binding in rat striatum, quantified using PET. Synapse 21: 45–53
Ito H, Hietala J, Blomqvist G, Halldin C, Farde L . (1998): Comparison of the transient equilibrium and continuous infusion method for quantitative PET analysis of [11C]raclopride binding. J Cereb Blood Flow Metab 18: 941–950
Ito K, Haga T, Lameh J, Sadee W . (1999): Sequestration of dopamine D2 receptors depends on coexpression of G-protein-coupled receptor kinases 2 or 5. Eur J Biochem 260: 112–119
Iwata SI, Izumi K, Nomoto M . (1992): Upregulation of postsynaptic dopamine receptors in the striatum does not influence haloperidol-induced catalepsy in mice. Pharmacol Biochem Behav 42: 803–808
Khan ZU, Gutierrez A, Martin R, Penafiel A, Rivera A, De La Calle A . (1998): Differential regional and cellular distribution of dopamine D2-like receptors: an immunocytochemical study of subtype-specific antibodies in rat and human brain. J. Comp Neurol 402: 353–371
Lammertsma AA, Hume SP . (1996): Simplified reference tissue model for PET receptor studies. Neuroimage 4: 153–158
Lammertsma AA, Bench CJ, Hume SP, Osman S, Gunn K, Brooks DJ, Frackowiak RSJ . (1996): Comparison of methods for analysis of clinical [11C]raclopride studies. J Cereb Blood Flow Metab 16: 42–52
Laruelle M . (2000): Imaging synaptic neurotransmission with in vivo binding competition techniques: A critical review. J Cereb Blood Flow Metab 20: 423–451
Laruelle M, D'Souza CD, Baldwin RM, Abi-Dargham A, Kanes SJ, Fingado CL, Seibyl JP, Zoghbi SS, Bowers MB, Jatlow P, Charney DS, Innis RB . (1997): Imaging D2 receptor occupancy by endogenous dopamine in humans. Neuropsychopharmacology 17: 162–174
Lewis DA, Melchitzky DS, Haycock JW . (1993): Four isoforms of tyrosine hydroxylase are expressed in human brain. Neuroscience 54: 477–492
Martres MP, Bouthenet ML, Sales N, Sokoloff P, Schwartz JC . (1985): Widespread distribution of brain dopamine receptors evidenced with [125I]iodosulpride, a highly selective ligand. Science 228: 752–755
McCann UD, Penetar DM, Shaham Y, Thorne DR, Gillin C, Sing HC, Thomas MA, Belenky G . (1992): Sleep deprivation and impaired cognition. Possible role of brain catecholamines. Biol Psychiatry 31: 1082–1097
McCann UD, Penetar DM, Shaham Y, Thorne DR, Sing HC, Thomas MA, Gillin C, Belenky G . (1993): Effects of catecholamine depletion on alertness and mood in rested and sleep deprived normal volunteers. Neuropsychopharmacology 8: 345–356
McNair DM, Lorr M, Droppleman LF . (1981): POMS Manual, 2nd ed. San Diego, CA, Educational and Industrial Testing Service
Meyer JH, Gunn RN, Myers R, Grasby PM . (1999): Assessment of spatial normalization of PET ligand images using ligand-specific templates. Neuroimage 9: 545–553
Mignot E, Laude D . (1985): Study of dopamine turnover by monitoring the decline of dopamine metabolites in rat CSF after alpha-methyl-p-tyrosine. J Neurochem 45: 1527–1533
Moresco RM, Loc'h C, Ottaviani M, Guibert B, Leviel V, Maziere M, Fazio F, Maziere B . (1999): Effects of dopamine on the in vivo binding of dopamine D2 receptor radioligands in rat striatum. Nucl Med Biol 26: 91–98
Narang N, Wamsley JK . (1995): Time dependent changes in DA uptake sites, D1 and D2 receptor binding and mRNA after 6-OHDA lesions of the medial forebrain bundle in the rat brain. J Chem Neuroanat 9: 41–53
Reitan RM, Wolfson D . (1985): The Halstead-Reitan Neuropsychological Test Battery. Tucson, AZ, Neuropsychology Press
Richfield EK, Young AB, Penney JB . (1986): Properties of D2 dopamine receptor autoradiography: High percentage of high-affinity agonist sites and increased nucleotide sensitivity in tissue sections. Brain Res 383: 121–128
Ross SB, Jackson DM . (1989): Kinetic properties of the accumulation of 3H-raclopride in the mouse brain in vivo. Arch Pharmacol 340: 6–12
Roy SD, McKay G, Hawes EM, Midha KK . (1984): Gas chromatographic quantitation of methoxyamphetamine and three of its metabolites in plasma. J Chromatogr 310: 307–317
Sassin JF, Frantz AG, Weitzman ED, Kapen S . (1972): Human prolactin: 24-hour pattern with increased release during sleep. Science 177: 1205–1207
Servan-Schreiber D, Carter CS, Bruno RM, Cohen JD . (1998): Dopamine and the mechanisms of cognition. II. D-amphetamine effects in human subjects performing a selective attention task. Biol Psychiatry 43: 723–729
Sibley DR, De Lean A, Creese I . (1982): Anterior pituitary dopamine receptors. Demonstration of interconvertible high and low affinity states of the D2 dopamine receptor. J Biol Chem 257: 6351–6361
Tabachnick BG, Fidell LS . (1996): Using Multivariate Statistics, 3rd ed. California State University, Northridge, Harper Collins College Publishers, pp 57–126
Takahashi S, Godse DD, Warsh JJ, Stancer HC . (1977): A gas chromatographic-mass spectrometric (GC-MS) assay for 3-methoxy-4-hydroxyphenylethyleneglycol and vanylmandelic acid in human serum. Clin Chim Acta 81: 183–192
Verhoeff NPLG . (1999): Radiotracer imaging of dopaminergic transmission in neuropsychiatric disorders. Psychopharmacology 147: 217–249
Volkow ND, Wang GJ, Fowler JS, Logan J, Schlyer D, Hitzemann R, Lieberman J, Angrist B, Pappas N, Burr G, Cooper T, Wolf AP . (1994): Imaging endogenous dopamine competition with [11C]raclopride in the human brain. Synapse 16: 255–262
Volkow ND, Gur RC, Wang GJ, Fowler JS, Moberg PJ, Ding YS, Hitzemann R, Smith G, Logan J . (1998): Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry 155: 344–349
Wang GJ, Volkow ND, Fowler JS, Wolf AP, MacGregor RR, Shea CE, Schlyer DJ, Hitzemann RJ . (1993): Comparison of two PET radioligands for imaging extrastriatal dopamine receptors in the human brain. Synapse 15: 246–249
Wang GJ, Volkow ND, Levy AV, Fowler JS, Logan J, Alexoff D, Hitzemann RJ, Schyler DJ . (1996a): MRI-PET image coregistration for quantification of striatal dopamine D2 receptors. J Comput Assist Tomogr 20: 423–428
Wang GJ, Volkow ND, Fowler JS, Logan J, Gur R, Netusil N, Hitzemann RJ, Pappas N . (1996b): Age associated decrements in dopamine D2 receptors in thalamus and in temporal insula of human subjects. Life Sci 59: PL31–PL35
Warsh JJ, Godse DD, Li PP . (1987): Quantitation of catecholamine metabolites by gas chromatography-mass spectrometry with selected ion monitoring. Methods Enzymol 142: 571–582
Weeks RA, Cunningham VJ, Piccini P, Waters S, Harding AE, Brooks DJ . (1997): 11C-diprenorphine binding in Huntington's disease: A comparison of region of interest analysis with statistical parametric mapping. J Cereb Blood Flow Metab 17: 943–949
Zimmermann RC, Krahn L, Klee G, Lu PY, Ory SJ, Lin SC . (1996): The impact of gender on alpha-methyl-para-tyrosine mediated changes in prolactin secretion and 6-hydroxymelatonin sulfate excretion. Psychoneuro-endocrinology 21: 469–478
Acknowledgements
This work was supported by a Young Investigator Award from the National Alliance for Research on Schizophrenia and Affective Disorders to Dr. Verhoeff, by a Postdoctoral Research Fellowship Award from the Ontario Mental Health Foundation to Dr. Verhoeff, and by a Clinician Scientist Award from the Medical Research Council of Canada to Dr. Kapur. The authors gratefully acknowledge Merck & Co., Inc., West Point, Pennsylvania, USA, and Merck Frosst Canada Inc., Point-Claire – Dorval, Quebec, Canada, for providing the AMPT; Anissa Abi-Dargham, M.D., Marc Laruelle, M.D., and Amit Anand, M.D., for sharing scientific information that made valuable contributions to the formulation of the study protocol; Alex Kecojevic, B.Sc., for assistance with subject recruitment; Colleen Millikin, M.Sc., for assistance with cognitive psychological testing; Terry Bell, Kevin Cheung, Steven Dobbin, and Erin Toole for assistance in PET data acquisition; Jean DaSilva, Ph.D., and Alan A. Wilson, Ph.D., for synthesis and plasma analysis of [11C]raclopride; Sylvain Houle, M.D., Ph.D., F.R.C.P.C., for providing the infrastructure enabling us to do the PET studies; Edward Dunn, Ph.D., F.C.A.C.B., for providing the infrastructure enabling us to do the laboratory studies; and Corey Jones, B.A., for the PET Centre computer system's management; staff of the Clinical Investigation Unit, 10th floor, Clarke Division, Centre for Addiction and Mental Health, for support for and management of the subjects during their overnight stay in the psychiatric inpatient unit.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Verhoeff, N., Kapur, S., Hussey, D. et al. A Simple Method to Measure Baseline Occupancy of Neostriatal Dopamine D2 Receptors by Dopamine In Vivo in Healthy Subjects. Neuropsychopharmacol 25, 213–223 (2001). https://doi.org/10.1016/S0893-133X(01)00231-7
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1016/S0893-133X(01)00231-7
Keywords
This article is cited by
-
Endogenous dopamine release in the human brain as a pharmacodynamic biomarker: evaluation of the new GPR139 agonist TAK-041 with [11C]PHNO PET
Neuropsychopharmacology (2022)
-
Estimating Endogenous Dopamine Levels at D2 and D3 Receptors in Humans using the Agonist Radiotracer [11C]-(+)-PHNO
Neuropsychopharmacology (2014)
-
The dopaminergic system in patients with functional dyspepsia analysed by single photon emission computed tomography (SPECT) and an alpha-methyl-para-tyrosine (AMPT) challenge test
European Journal of Nuclear Medicine and Molecular Imaging (2012)
-
Effects of antipsychotic treatment on psychopathology and motor symptoms. A placebo-controlled study in healthy volunteers
Psychopharmacology (2011)
-
Model-based parametric study of frontostriatal abnormalities in schizophrenia patients
BMC Psychiatry (2010)