Abstract
In unilaterally 6-hydroxydopamine (6-OHDA)-lesioned rats, a rodent model of Parkinson's disease (PD), the adenosine A2A receptor antagonist SCH 58261 significantly increased (+180%, p < .01) the number of rotations induced by a low dose of quinpirole (a dopamine D2 receptor agonist), while it did not significantly modify the effects of a comparably low dose of SKF 38393 (a dopamine D1 receptor agonist). The dose-dependent potentiating effects of SCH 58261 on quinpirole-induced turning were similar in caffeine-treated rats (1 g/l in drinking water over 14 days) and control animals (tap water). The selective potentiating effects of SCH 58261 on D2-dependent turning confirm the existence of a potent and specific A2A/D2 receptor-receptor interaction. The persistence of the potentiating effects of SCH 58261 after chronic caffeine intake suggests that no tolerance should develop towards the antiparkinsonian effects of adenosine A2A receptor antagonists with chronic treatment.
Similar content being viewed by others
Main
The two major output pathways from the basal ganglia, the direct (striato-nigral) and the indirect (striato-pallidal) pathways, have opposing effects on thalamocortical neurones and subsequently on motor activity (Alexander and Crutcher 1990). A co-ordinated balance of the pathway functions results in normal movements, while predominance of the indirect or the direct pathways is thought to underlie hypo- and hyper-kinetic disorders, respectively (Albin et al. 1989). For instance, over-activity of the indirect pathway (due to the reduction of dopamine D2 receptor-mediated inhibition of striato-pallidal neurones) is thought to determine the motor deficits of Parkinson's disease (PD) (Alexander and Crutcher 1990; Gerfen et al. 1990).
In the striato-pallidal neurones, dopamine D2 receptors are co-localized with adenosine A2A receptors (Fink et al. 1992; Schiffmann et al. 1991; Svenningsson et al. 1997a), and a tonic, antagonistic A2A/D2 interaction has been reported. The stimulation of A2A receptors decreases the affinity of D2 receptors for the agonist in rat striatal membranes (Ferré et al. 1991b) and in a mouse fibroblast cell line stably cotransfected with A2A and D2 receptors (Dasgupta et al. 1996). Adenosine A2A receptor agonists inhibit, while A2A receptor antagonists potentiate, the effects of D2 receptor agonists on motor activity, neurotransmitter release and striatal c-Fos expression (Ferré et al. 1991a, 1993, 1999; Jin et al. 1993; Morelli et al. 1995; Pollack and Fink 1995). While much data indicates that A2A receptors selectively interact with dopamine D2 receptors (reviewed in Ferré et al. 1997), adenosine A2A receptor blockade has been reported to potentiate both D1- and D2-dependent contralateral rotations in 6-hydroxydopamine (6-OHDA)-lesioned rats (Fenu et al. 1997; Pinna et al. 1996). One of the possible explanations for these findings is a synergistic D1-D2 interaction occurring due to a direct stimulation of D1 receptors (by the D1 receptor agonist) and a facilitation of D2 receptors (by the removal of a tonic A2A-dependent inhibition). If A2A receptor blockade directly potentiated D2- mediated responses while only indirectly potentiated D1-mediated effects, we would expect the effects elicited by very low doses of D1 and D2 receptor agonists to be modulated differently by A2A receptor blockade.
Due to the key role played by adenosine A2A receptors in the regulation of striatal dopaminergic neurotransmission, drugs acting on these receptors are likely to be useful in the treatment of neurological disorders related to dopaminergic dysfunction (Ferré et al. 1992; Ongini and Fredholm 1996). In particular, adenosine A2A receptor blockade is likely to counteract (through the potentiation of D2 receptor-mediated effects) the over-activity of the indirect pathway in PD. In fact, adenosine A2A receptor antagonists have recently been proposed as potential new agents for treating this disease (Richardson et al. 1998). The current PD treatment is based almost entirely on dopamine replacement therapy using L-dopa but the occurrence of dyskinesia and/or psychosis and the loss of efficacy over time greatly impair the therapeutic benefit of this drug. Although adenosine A2A receptor antagonists may be a very promising therapeutic alternative, tolerance may develop due to chronic administration. In fact chronic treatment with caffeine, a non-selective adenosine receptor antagonist, has been shown to induce tolerance to the motor stimulating effects of caffeine in rodents (Garrett and Holtzman 1995a,b; Nikodijevic et al. 1993a,b), and to caffeine-induced c-Fos expression in the rat globus pallidus (Svenningsson and Fredholm 1997).
The first aim of this paper was to evaluate whether SCH 58261, a selective adenosine A2A receptor antagonist (Zocchi et al. 1996), influenced differently the turning behavior elicited by comparably low doses of D1 (SKF 38393) and D2 (quinpirole) receptor agonists in rats unilaterally lesioned with 6-OHDA, a rodent model of PD (Schwarting and Huston 1996; Ungerstedt 1971). Secondly, we aimed to evaluate whether the potentiating effects of SCH 58261 were reduced by chronic adenosine receptor blockade.
METHODS
Experimental Protocol
Male Sprague-Dawley rats (145-155 g) were used. The animals were kept under standardized temperature, humidity and lighting conditions, with free access to water and food. Animal care and use followed the directives of the Council of the European Communities (86/609/EEC). Under Equithesin (3 ml/kg) anaesthesia, animals were placed in a Kopf stereotaxic apparatus. Unilateral injections of 6-hydroxydopamine (8μg / 4μl of 0.2% ascorbic acid saline solution) were performed in the left nigrostriatal pathway (coordinates with respect to bregma: A = −2.4; L = +1.2; V = −7.8 mm) by means of a Hamilton syringe (mod. 701). Starting 3 weeks after the lesion, the animals' ability to rotate in response to apomorphine (0.05 mg/kg subcutaneously) was tested. Contralateral rotations induced by apomorphine were measured 3-4 times at weekly intervals. Only animals showing at least 50 turns/ 5 min in the last test were included in the study. Thirty minutes prior to starting the experiments, the animals were placed in rotation bowls in a soundproof experimental room. Each trial was analysed by an observer unaware of the treatment received by the animals. Only complete (360°) and uninterrupted turns were recorded over 60 min.
Dose-response effects of Dopamine D1 and D2 Receptor Agonists
The ability of different doses of SKF 38393 and quinpirole to induce contralateral rotations was evaluated in groups of 6-8 lesioned rats each. Low doses of both drugs (1/5 of the minimal dose inducing a maximal effect) were selected for the subsequent experiments aimed at evaluating the potentiating effects of SCH 58261.
Influence of SCH 58261 on Quinpirole- and SKF 38393-Induced Turning
Either quinpirole (0.02 mg/kg) or SKF 38393 (0.6 mg/kg) were administered intraperitoneally (i.p.) 15 min after SCH 58261 (2 mg/kg i.p.) or vehicle (n = 8 in both groups). The dose of SCH 58261 used in these experiments was selected on the basis of a previous study (Popoli et al. 1998).
Chronic Caffeine Treatment
To test whether chronic caffeine intake affected the ability of SCH 58261 to potentiate quinpirole-induced rotations, the effects of three different doses of SCH 58261 were tested in both caffeine-treated and control rats. A caffeine solution (1 g/l in drinking water) was administered over 14 consecutive days to lesioned rats. The dose of caffeine was chosen on the basis of previous reports (Garrett and Holtzman 1995a, b; Holtzman et al. 1991). Animals had free access to drinking bottles over the entire period. The daily caffeine intake was determined twice a week by weighing each bottle on consecutive days at 9.30 A.M.; a difference of 1 g in weight was assumed to represent 1 ml of caffeine solution consumed. Control rats received normal drinking water. At the end of chronic treatment, both caffeine-treated and control rats received SCH 58261 (0, 0.5, 1 and 2 mg/kg i.p.) plus quinpirole (0.02 mg/kg). Each group was made up of eight animals.
Drugs
Anhydrous caffeine base (Sigma) was dissolved in tap water. (±)-SKF 38393 hydrochloride ((±)-1-Phenyl-2,3,4,5-tetrahydro-(1H)-3-benzazepine-7,8-diol hydrochloride, RBI) and (-)Quinpirole hydrochloride (RBI) were dissolved in distilled water. SCH 58261 {7-(2-phenylethyl)-5-amino-2-(2-furyl)-pyrazolo-[4,3-e]-1,2,4-triazolo[1,5c]pyrimidine} (Schering Plough, Milan, Italy) was dissolved in dimethyl-sulfoxide (DMSO). CPT (8-Cyclopenthyltheophylline, RBI), was dissolved in water with a minimal amount of 0.1N NaOH.
Statistics
One-way ANOVA followed by Dunnett's test, and Student's t test were used to assess the statistical significance between groups.
RESULTS
Dose-Response Curves of Dopamine D1 and D2 Receptor Agonists
Both quinpirole and SKF 38393 (Figure 1) induced dose-dependent contralateral rotations in 6-OHDA-lesioned rats. The minimal doses inducing a maximal effect were 0.1 mg/kg of quinpirole and 3 mg/kg of SKF 38393.
Influence of SCH 58261 on the Turning Behavior Elicited by Low Doses of D1 and D2 Receptor Agonists
The adenosine A2A receptor antagonist SCH 58261 (2 mg/kg) potentiated significantly the contralateral rotations induced by a low dose (0.02 mg/kg) of quinpirole (Figure 2). On the contrary, the effects of a low dose (0.6 mg/kg) of SKF 38393 were not significantly potentiated by SCH 58261 (Figure 2). In order to verify whether the lack of potentiating effects of SCH 58261 towards SKF 38393-induced rotations could merely be ascribed to the high inter-individual variability (as suggested by the large S.E.M) of the effects elicited by this dose of SKF 38393, two additional sets of experiments were performed. First, the ability of N6-cyclopenthyl-theophylline (CPT, a selective adenosine A1 receptor antagonist) to potentiate the turning behavior induced by SKF 38393 0.6 mg/kg was tested in 6 rats. CPT (7.2 mg/kg, 15 min before) increased significantly the number of contralateral rotations induced by SKF 38393 (Figure 3, left). In a second series of experiments, the influence of SCH 58261 on the turning behavior induced by a higher dose (1.2 mg/kg) of SKF 38393 was studied. SCH 58261 was unable to potentiate significantly the turning induced by SKF 38393 1.2 mg/kg (Figure 2). Neither SCH 58261 2 mg/kg nor CPT 7.2 mg/kg induced turning when administered alone (n=5 in each group, data not shown).
Influence of SCH 58261 on the turning behaviour induced by quinpirole (0.02 mg/kg) and SKF 38393 (0.6 and 1.2 mg/kg) in 6-hydroxydopamine-lesioned rats. Either quinpirole or SKF 38393 were administered 15 min after SCH 58261 (2 mg/kg) or vehicle. Each group was composed of 6–8 animals. *p = .01 versus quinpirole (Student's t test)
Influence of N6-cyclopenthylthoephylline (CPT, 7.2 mg/kg) on the turning behaviour induced by SKF 38393 (0.6 mg/kg) in control and caffeine-treated 6-hydroxydo-pamine-lesioned rats. Left: (“control”): CPT (7.2 mg/kg, 15 min before) significantly potentiated SKF 38393-induced turning in control rats. Right: “chronic caffeine”): rats received caffeine (1 g/l in their drinking water) over 14 days. At the end of chronic treatment, they were treated with SKF 38393 alone (0.6 mg/kg) or CPT (7.2 mg/kg) plus SKF 38393. Each group was composed of six animals. * = p < .005 versus SKF 38393 alone according to Student's t test
Chronic Caffeine Treatment
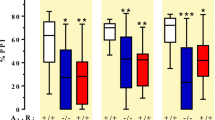
In rats treated chronically with caffeine, an average daily intake of 65 mg/kg was estimated. The average body weight of caffeine-treated rats was comparable to the average weight of rats in the control group. In both caffeine-treated and control rats, SCH 58261 potentiated quinpirole-induced turning in a dose-dependent way, the dose-response effects of SCH 58261 being very similar in the two groups (Figure 4). At a dose of 2 mg/kg, SCH 58261 increased significantly quinpirole-induced rotations (+180% and 175% in caffeine-treated and control rats, respectively), while a non significant potentiation was observed with a dose of 1 mg/kg.
Dose-response effects of SCH 58261 on quinpirole-induced turning in control and caffeine-treated rats. A) Control rats (which had received normal drinking water over 14 days) were treated with vehicle or SCH 58261 0.5, 1 and 2 mg/kg plus (15 min thereafter) quinpirole 0.02 mg/kg. B) Caffeine-treated rats received caffeine (1 g/l in their drinking water) over 14 days. At the end of chronic treatment, they received vehicle or SCH 58261 0.5, 1 and 2 mg/kg plus (15 min thereafter) quinpirole 0.02 mg/kg. Each group was composed of eight animals. * = p < .05 versus quinpirole (One-way ANOVA followed by Dunnett's test)
The number of contralateral rotations induced by quinpirole 0.02 mg/kg was not reduced by chronic caffeine intake. Since chronic caffeine treatment had been previously reported to induce tolerance to the effects of quinpirole in 6-OHDA-lesioned rats (Garrett and Holtzman 1995b), additional experiments were performed to verify whether tolerance to the effects of a fully-effective dose of quinpirole developed. Two groups of 8 rats each were treated with quinpirole 0.1 mg/kg after having received caffeine or drinking water for 14 days. The number of quinpirole-induced contralateral rotations was reduced (-40%; p < .05 according to Student's t test) in caffeine-treated rats with respect to controls (data not shown).
Finally, in order to verify whether our “chronic” protocol was actually suitable to induce tolerance to the motor effects of selective adenosine receptor antagonists, the influence of CPT on SKF 38393-induced turning was also tested in a separate group of 6 caffeine-treated rats. Chronic caffeine intake abolished the potentiating effects of the selective adenosine A1 receptor antagonist (Figure 3, right).
DISCUSSION
Influence of SCH 58261 on D1- and D2- Dependent Turning Behavior
In the present experiments, SCH 58261 potentiated significantly the effects of a low dose of quinpirole whereas it did not modify significantly the effects of a comparably low dose of SKF 38393. Although 0.02 mg/kg of quinpirole and 0.6 mg/kg of SKF 38393 induced approximately the same average number of rotations, the effects of SKF 38393 showed a greater inter-individual variability. This might indicate that the motor effects elicited by 0.6 mg/kg of SKF 38393 are poorly susceptible to potentiation irrespective of the treatment. However, the turning behavior induced by SKF 0.6 mg/kg could be markedly and significantly potentiated by the selective adenosine A1 receptor antagonist CPT. Moreover, even the effects induced by a higher dose (1.2 mg/kg) of SKF 38393 were not potentiated by SCH 58261. This latter finding agrees with a previous report which shows the ineffectiveness of SCH 58261 (3 mg/kg) in potentiating the turning behavior induced by SKF 38393 1.5 mg/kg (Pinna et al. 1996). The finding that, at least in a particular range of doses, D1-dependent turning behavior is not potentiated by adenosine A2A receptor blockade does not support the view that a direct interaction between A2A and D1 receptors exists. Indeed, there is no direct evidence of a co-localization of these two receptors (Svenningsson et al. 1997a). Assuming that A2A and D1 receptors do not interact directly, the previously reported potentiating effects of A2A receptor antagonists on D1-mediated rotations (Pinna et al. 1996) could depend on the disinhibition of D2-dependent response, leading to a D1-D2 synergistic activation. Some evidence, however, suggests that this is not the only possible mechanism of the reported A2A/D1 interaction. First, in 6-OHDA-lesioned rats the pattern of striatal c-Fos expression following coadministration of a D1 agonist and an adenosine antagonist was different from the c-Fos expression pattern observed following co-administration of D1 and D2 agonists (Pollack and Fink 1996). Moreover, the “synergistic” hypothesis can not explain the inhibitory effects exerted by certain doses of an A2A receptor agonist on both D1- and D2-induced turning (Morelli et al. 1994). Since adenosine A2A receptors modulate acetylcholine (Brown et al. 1990), GABA (Ferré et al. 1993) and glutamate release (Popoli et al. 1995), other indirect mechanisms may also account for the A2A/D1 interaction.
As for the ability of SCH58261 to selectively potentiate D2-dependent rotations, this finding is in line with a previous study showing that CGS 21680, an adenosine A2A receptor agonist, antagonized quinpirole- but not SKF 38393-induced turning in 6-OHDA-lesioned rats (Ferré et al. 1999). Taken together, these results provide a behavioral support for the view that a specific antagonistic interaction exists between adenosine A2 and dopamine D2 receptors in the neostriatum (Ferré et al. 1991b), an interaction which was found to be even increased in 6-OHDA-lesioned rats (Ferré and Fuxe 1992).
In agreement with previous studies (Pinna et al. 1996), neither SCH 58261 nor CPT induced rotational behavior in themselves in 6-OHDA-lesioned rats. This finding, which is apparently at odds with the ability of non-selective adenosine receptor antagonists, such as caffeine, to induce turning behavior in the same model, may indicate that in the denervated striatum the residual dopaminergic tone at D1 and D2 receptors is too low to become enhanced by selective A1 and A2A receptor blockade, respectively.
Influence of Chronic Caffeine Intake on the Effects of SCH 58261
One of the aims of the present study was to verify whether the ability of SCH 58261 to potentiate quinpirole-induced rotations in a rodent model of PD was reduced by chronic caffeine treatment. The best way to investigate whether tolerance could develop towards the effects of a selective A2A receptor antagonist would have been to use SCH 58261, instead of the non-selective antagonist caffeine, in chronic treatment. Unfortunately, SCH 58261 is not water-soluble, and performing daily injections of a DMSO solution would have been unethical due to the related irritating properties. On the other hand, since the effects of caffeine seem to mainly depend on the blockade of adenosine A2A receptors (Svenningsson et al. 1997b), caffeine may be considered an acceptable alternative to an A2A antagonist.
Although it was only possible to estimate the daily intake of caffeine, the mean caffeine intake noted here is similar to that reported by other authors who had used a scheduled access protocol (Holtzman et al. 1991; Garrett and Holtzman 1994, 1995b). The development of tolerance to the effects elicited by quinpirole 0.1 mg/kg after chronic caffeine is also in accordance with a previous report (Garrett and Holtzman 1995b). Moreover, the finding that chronic caffeine intake abolishes the potentiating effects of CPT indicates that the experimental protocol used here was suitable for the purposes of the study.
The similarity of the dose-response curves of SCH 58261 plus quinpirole in caffeine-treated and control animals indicate that chronic adenosine receptor blockade does not induce tolerance to the potentiating effects of SCH 58261. Conversely, tolerance developed to the potentiating effects of an adenosine A1 receptor antagonist. This differential influence of chronic caffeine intake on the effects of A1 (tolerance) and A2A (no tolerance) receptor antagonists is in line with several reports showing that A1, but not A2A receptors, are up-regulated after chronic caffeine (reviewed in Jacobson et al. 1996). Moreover, although the motor effects of caffeine appear to depend mainly on the blockade of adenosine A2A receptors (Svenningsson et al. 1997b), such receptors seem to be not involved primarily in the development of tolerance to the motor stimulating effects of caffeine after chronic administration (Garrett and Holtzman 1995a,b; Holtzman et al. 1991; Nikodijevic et al. 1993a,b).
The present finding of persistent potentiating effects of SCH 58261 after chronic adenosine receptor blockade is in full agreement with a recent report showing that the ability of KW-6002 (a selective A2A receptor antagonist) to reverse motor defects in parkinsonian monkeys was maintained after chronic administration over 21 days (Kanda et al. 1998).
In conclusion, these results confirm that a potent and selective A2A/D2 interaction exists, and support the hypothesis that A2A receptor blockade could be a suitable alternative approach to the treatment of PD.
References
Albin RL, Young AB, Penney JB . (1989): The functional anatomy of basal ganglia disorders. Trends Neurosci 12: 366–375
Alexander GR, Crutcher MD . (1990): Functional architecture and basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci 13: 266–271
Brown SJ, James S, Reddington M, Richardson PJ . (1990): Both A1 and A2a purine receptors regulate striatal acetylcholine release. J Neurochem 55: 31–37
Dasgupta S, Ferré S, Kull B, Hedlund PB, Finnman UB, Ahlberg S, Arenas E, Fredholm BB, Fuxe K . (1996): Adenosine A2A receptor modulate the binding characteristics of dopamine D2 receptors in stably cotransfected fibroblast cells. Eur J Pharmacol 316: 325–331
Fenu S, Pinna A, Ongini E, Morelli M . (1997): Adenosine A2A receptor antagonism potentiates L-DOPA-induced turning behaviour and c-fos expression in 6-hydroxydopamine-lesioned rats. Eur J Pharmacol 321: 143–147
Ferré S, Herrera-Marschitz M, Grabowska-Andén M, Casa M, Ungerstedt U, Andén NE . (1991a): Postsynaptic dopamine/adenosine interaction. I. Adenosine analogues inhibit dopamine D 2-mediated behaviour in short-term reserpinized mice. Eur J Pharmacol 192: 25–29
Ferré S, Von Euler G, Johansson B, Fredholm B, Fuxe K . (1991b): Stimulation of high affinity adenosine A-2 receptors decreases the affinity of dopamine D-2 receptors in rat striatal membranes. Proc Natl Acad Sci USA 88: 7238–7241
Ferré S, Fuxe K . (1992): Dopamine denervation leads to an increase in the intramembrane interactions between adenosine A2 and dopamine D2 receptors in the neostriatum. Brain Res 594: 124–130
Ferré S, Fuxe K, Von Euler G, Johansson B, Fredholm BB . (1992): Adenosine-dopamine interactions in the brain. Neuroscience 51: 501–512
Ferré S, O'Connor WT, Fuxe K, Ungerstedt U . (1993): The striopallidal neuron: a main locus for adenosine-dopamine interactions in the brain. J Neurosci 13: 5402–5406
Ferré S, Fredholm BB, Morelli M, Popoli P, Fuxe K . (1997): Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci 20: 482–487
Ferré S, Popoli P, Rimondini R, Reggio R, Kehr J, Fuxe K . (1999): Adenosine A2A and group I metabotropic glutamate receptors synergistically modulate the binding characteristics of dopamine D2 receptors in the rat striatum. Neuropharmacol 38: 129–140
Fink JS, Weaver DR, Rivkees SA, Peterfreund RA, Pollack A, Adler DM, Reppert SM . (1992): Molecular cloning of the rat A2 adenosine receptors: selective co-expression with D2 dopamine receptors in rat striatum. Mol Brain Res 14: 186–195
Garrett BE, Holtzman SG . (1994): Caffeine cross-tolerance to the selective dopamine D1 and D2 receptor agonists but not to their synergistic interaction. Eur J Pharmacol 262: 65–75
Garrett BE, Holtzman SG . (1995a): Does adenosine receptor blockade mediate caffeine-induced rotational behaviour?. J Pharmacol Exp Ther 274: 207–214
Garrett BE, Holtzman SG . (1995b): The effects of dopamine agonists on rotational behavior in non-tolerant and caffeine-tolerant rats. Behav Pharmacol 6: 843–851
Gerfen CR, Engber TM, Mahan L, Susel Z, Chase TN, Monsma FJ, Sibley DR . (1990): D1 and D2 dopamine receptor-regulated gene expression of strionigral and striopallidal neurons. Science 250: 1429–1432
Holtzman SG, Mante S, Minneman KP . (1991): Role of adenosine receptors in caffeine tolerance. J Pharmacol Exp Ther 256: 62–68
Kanda T, Jackson MJ, Smith LA, Pearce RKB, Nakamura J, Kase H, Kuwana Y, Jenner P . (1998): Adenosine A2A antagonist: a novel antiparkinsonian agent that does not provoke dyskinesia in parkinsonian monkeys. Ann Neurol 43: 507–513
Jacobson KA, Von Lubitz DKJE, Daly JW, Fredholm BB . (1996): Adenosine receptor ligands: diferences with acute versus chronic treatment. Trends Pharmacol Sci 17: 108–113
Jin S, Johansson B, Fredholm BB . (1993): Effects of adenosine A1 and A2 receptor activation on electrically evoked dopamine and acetylcholine release from rat striatal slices. J Pharmacol Exp Ther 267: 801–808
Morelli M, Fenu S, Pinna A, Di Chiara G . (1994): Adenosine A2 receptors interact negatively with dopamine D1 and D2 receptors in unilaterally 6-hydroxydopamine-lesioned rats. Eur J Pharmacol 251: 21–25
Morelli M, Pinna A, Wardas J, Di Chiara G . (1995): Adenosine A2 receptors stimulate c-fos expression in striatal neurons of 6-hydroxydopamine-lesioned rats. Neuroscience 67: 49–55
Nikodijevic O, Jacobson KA, Daly JW . (1993a): Locomotor activity in mice during chronic treatment with caffeine and withdrawal. Pharmacol Biochem Behav 44: 199–216
Nikodijevic O, Jacobson KA, Daly JW . (1993b): Effects of combinations of methylxanthines and adenosine analogs on locomotor activity in control and chronic caffeine-treated mice. Drug Dev Res 30: 104–110
Ongini E, Fredholm BB . (1996): Pharmacology of adenosine A2A receptors. Trends Pharmacol Sci 17: 364–372
Pinna A, Di Chiara G, Wardas J, Morelli M . (1996): Blockade of A2a receptors positively modulates turning behaviour and c-Fos expression induced by D1 agonists in dopamine-denervated rats. Eur J Neurosci 8: 1176–1181
Pollack AE, Fink JS . (1995): Adenosine antagonists potentiate D2-dopamine-dependent activation of Fos in the striopallidal pathway. Neuroscience 68: 721–728
Pollack AE, Fink JS . (1996): Synergistic interaction between an adenosine antagonist and a D1 dopamine agonist on rotational behavior and striatal c-Fos induction in 6-hydroxydopamine-lesioned rats. Brain Res 743: 124–130
Popoli P, Betto P, Reggio R, Ricciarello G . (1995): Adenosine A2A receptor stimulation enhances striatal extracellular glutamate levels in rats. Eur J Pharmacol 287: 215–217
Popoli P, Reggio R, Pèzzola A, Fuxe K, Ferré S . (1998): Adenosine A1 and A2 receptor antagonists stimulate motor activity: evidence for an increased effectiveness in aged rats. Neurosci Lett 251: 201–204
Richardson PJ, Kase H, Jenner PG . (1998): Adenosine A2A receptor antagonists as new agents for the treatment of Parkinson's disease. Trends Pharmacol Sci 18: 338–344
Schiffmann SN, Jacobs O, Vanderhaeghen JJ . (1991): Striatal restricted adenosine A2 receptor (RDC8) is expressed by enkephalin but not by substance P neurons: an in situ hybridization histochemistry study. J Neurochem 57: 1062–1067
Schwarting PKW, Huston JP . (1996): The unilateral 6-hydroxydopamine lesion model in behavioural brain research. Analysis of functional deficits, recovery and treatments. Progr Neurobiol 50: 275–331
Svenningsson P, Fredholm BB . (1997): Caffeine mimics the effect of a dopamine D2/3 receptor agonist on the expression of immediate early genes in globus pallidus. Neuropharmacol 36: 1309–1317
Svenningsson P, Le Moine C, Kull B, Sunahara R, Bloch B, Fredholm BB . (1997a): Cellular expression of adenosine A2A receptor messenger RNA in the rat central nervous system with special reference to dopamine innervated areas. Neuroscience 80: 1171–1185
Svenningsson P, Nomikos G, Ongini E, Fredholm BB . (1997b): Antagonism of adenosine A2A receptors underlies the behavioural activating effect of caffeine and is associated with reduced expression of messenger RNA for NGFI-A and NGFI-B in caudate-putamen and nucleus accumbens. Neuroscience 79: 753–764
Ungerstedt U . (1971): Postsynaptic supersensitivity after 6-hydroxydopamine induced degeneration of the nigrostriatal dopamine system in the rat brain. Acta Physiol Scand 82 Suppl. 367: 69–93
Zocchi C, Ongini E, Conti A, Monopoli A, Negretti A, Baraldi PG, Dionisotti S . (1996): The non-xanthine heterocyclic compound SCH 58261 is a new potent and selective A2a adenosine receptor antagonist. J Pharmacol Exp Ther 276: 398–404
Acknowledgements
The authors thank Prof. Kjell Fuxe and Dr. Sergi Ferré (Department of Neuroscience, Karolinksa Institute, Stockholm, Sweden) for helpful discussions throughout the study and Dr. Ennio Ongini (Schering Plough, Milan) for generously providing SCH 58261.
This work was supported by a coordinated European BIOMED 2 project (BM4-CT96-0238).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Popoli, P., Reggio, R. & Pèzzola, A. Effects of SCH 58261, an Adenosine A2A Receptor Antagonist, on Quinpirole-Induced Turning in 6-Hydroxydopamine-Lesioned Rats: Lack of Tolerance after Chronic Caffeine Intake. Neuropsychopharmacol 22, 522–529 (2000). https://doi.org/10.1016/S0893-133X(99)00144-X
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1016/S0893-133X(99)00144-X
Keywords
This article is cited by
-
Brain activity during a working memory task after daily caffeine intake and caffeine withdrawal: a randomized double-blind placebo-controlled trial
Scientific Reports (2023)
-
Molecular and Structural Insight into Adenosine A2A Receptor in Neurodegenerative Disorders: A Significant Target for Efficient Treatment Approach
Molecular Neurobiology (2023)
-
Exploration of chalcones and related heterocycle compounds as ligands of adenosine receptors: therapeutics development
Molecular Diversity (2022)
-
Mechanisms of the psychostimulant effects of caffeine: implications for substance use disorders
Psychopharmacology (2016)
-
Adenosine A1 receptor: Functional receptor-receptor interactions in the brain
Purinergic Signalling (2007)