Abstract
We have recently shown that priming injections of alcohol and footshock stress reinstate alcohol seeking in drug-free rats. Here we tested whether naltrexone and fluoxetine, two drugs used in the treatment of alcohol dependence, would affect reinstatement of alcohol seeking induced by these events. We also determined the effects of these drugs on alcohol self-administration during the maintenance phase. Rats were trained to press a lever for a 12% w/v alcohol solution. After stable drug-taking behavior was obtained, lever pressing for alcohol was extinguished. Reinstatement of drug seeking was then determined after priming injections of alcohol (0.24–0.96 g/kg) or exposure to intermittent footshock (5 and 15 min). Rats were pretreated with naltrexone (0.2–0.4 mg/kg) or fluoxetine (2.5–5 mg/kg) during maintenance or during tests for reinstatement. Both naltrexone and fluoxetine decreased lever presses for alcohol during the maintenance phase. Naltrexone blocked alcohol-induced, but not stress-induced reinstatement. In contrast, fluoxetine blocked stress-induced reinstatement, while its effect on alcohol-induced reinstatement was less consistent. The implications of these data to the understanding of relapse to alcohol are discussed.
Similar content being viewed by others
Main
Studies with laboratory rats have shown mu opioid antagonists and selective serotonin reuptake inhibitors (SSRIs) decrease alcohol self-administration in a number of experimental procedures (Amit et al. 1991; Herz 1997; Lê et al. 1996). The preferentially mu opioid receptor antagonist, naltrexone, and SSRI agents such as fluoxetine also have been shown to decrease relapse to alcohol in humans (Naranjo and Sellers 1989; O'Malley et al. 1992; Sellers et al. 1992; Volpicelli et al. 1992). It is important to note, however, that several studies failed to find that SSRIs decrease rates of relapse (Zernig et al. 1997). In addition, although naltrexone has been found to decrease rates of relapse in alcoholics, a high proportion of these individuals relapse to alcohol during naltrexone treatment (Volpicelli et al. 1997). Thus, it appears that while drugs such as naltrexone and fluoxetine consistently decrease alcohol consumption in laboratory animals, their clinical efficacy in humans is more variable.
One important difference between the studies with humans versus the studies with laboratory animals is that those with humans concentrated on the relapse phase, while those with rats were done during the maintenance phase of the addiction process. Consequently, data on the effect of fluoxetine and naltrexone on relapse to alcohol seeking in preclinical models do not exist. In the present study, therefore, we used a reinstatement procedure, an animal model of relapse (Carroll and Comer 1996; Stewart and de Wit 1987), to study the effect of fluoxetine and naltrexone on relapse to drug seeking induced by reexposure to alcohol and exposure to a footshock stressor. Acute reexposure to alcohol (Bigelow et al. 1977; de Wit 1996; de Wit and Chutuape 1993; Hodgson et al. 1979; Ludwig et al. 1974) and exposure to stress (Brown et al. 1995; Cooper et al. 1992; Hore 1971) are regarded as two important factors for provoking relapse in humans.
We have recently modified the reinstatement method, previously used to study factors involved in relapse to opioid and stimulant drugs in rats and monkeys, in order to determine factors involved in relapse to alcohol seeking in rats (Lê et al. 1998). We found that priming injections of alcohol produce a modest dose-dependent reinstatement of drug seeking, whereas footshock stress potently reinstates extinguished alcohol seeking. These data parallel those obtained in studies with heroin- and cocaine-trained rats (Erb et al. 1996; Shaham and Stewart 1995).
In the present series of studies, rats were initially given access to alcohol in a two-bottle limited access procedure. Subsequently, rats were trained in operant chambers to lever press for a 12% alcohol solution. After stable drug-taking behavior was observed, the drug-reinforced behavior was extinguished by terminating alcohol delivery. Subsequently, the rats were pretreated with naltrexone or fluoxetine and tested for reinstatement of alcohol seeking induced by priming injections of alcohol and exposure to an intermittent footshock stressor. Different groups of rats were also tested for the effect of naltrexone and fluoxetine on alcohol self-administration during the maintenance phase.
MATERIAL AND METHODS
Subjects
Male Wistar rats (Charles River, Montreal; about 150–200 g at the start of the experiments) were individually housed with food and water available ad libitum. The temperature was maintained at 21 ± 1°C and lights were on from 7 a.m. to 7 p.m. Rats were initially trained to consume alcohol in a limited access procedure (Linseman 1987). Briefly, rats were provided with access to an alcohol solution and water in modified “Richter” tubes for 30 min/day in drinking cages. Alcohol solutions were provided in escalating concentrations: 3% w/v for the first 8 days, 6% for the next 10 days, and 12% for the final 12 days. Rats that consumed less than 0.4 g/kg over a period of 30 min during the 12% phase were eliminated from the experiments. All procedures were done in accordance with the guidelines of the CCAC and were approved by the local Animal Care Committee.
Apparatus
Sixteen operant chambers were used. Each chamber was equipped with two levers, symmetrically centred on the side panel. Responding on one lever (an active lever) activated the infusion pump (Razel Sci., Stamford, CT). Presses on the other lever (an inactive lever) were recorded, but did not activate the pump. Activation of the infusion pump resulted in a delivery of 0.19 ml of a 12 % w/v alcohol solution to a liquid drop receptacle located between the two levers over a period of 5 sec. During the infusion, a stimulus light above the active lever was turned on for 6 sec. Lever presses during this timeout period were counted, but did not lead to further infusions.
Drugs
Naltrexone (Dupont-Merck) and fluoxetine (a gift from Eli Lilly) were dissolved in physiological saline and injected at a volume of 1 ml/kg. Naltrexone was injected SC (15-minute pretreatment time) and fluoxetine was injected IP (30-minute pretreatment time) before the start of the sessions during the maintenance phase or before exposure to alcohol priming or footshock during the tests for reinstatement.
STUDY 1: Maintenance
Operant self-administration of alcohol was initiated on a fixed ratio-1 schedule (FR-1, each lever press is reinforced) with a 6 sec timeout period after each delivery of the reinforcer (0.19 ml of a 12% alcohol solution). The self-administration sessions were carried out for 60 min/day, 7 days/week. The experiments were carried out for 7 days/week in order to avoid the “alcohol deprivation effect” (Sinclair and Senter 1967). Responding on the FR-1 schedule was maintained for 10 sessions. The requirement for alcohol delivery was then increased to a FR-2 schedule for 4–5 sessions. Subsequently, the schedule requirement was increased to a FR-3 schedule until the rats obtained 3 days of stable drug taking (less than 20% deviation from the mean).
Experiment 1—Maintenance: Naltrexone
After obtaining stable drug-taking behavior, the rats were divided into two groups. One group was pretreated with saline and the other group was pretreated with naltrexone (n = 7 per group). The rats pretreated with naltrexone were given the drug for 4 consecutive days at a dose of 0.2 mg/kg and for 4 additional days at a dose of 0.4 mg/kg. Vehicle-treated rats were given saline injections during the 8-day period.
Experiment 2—Maintenance: Fluoxetine
After obtaining stable drug-taking behavior, the rats were divided into two groups. One group was pretreated with saline and the other group was pretreated with fluoxetine (n=8 per group). The rats pretreated with fluoxetine were given the drug for 4 consecutive days at a dose of 2.5 mg/kg and for 4 additional days at a dose of 5 mg/kg. Vehicle-treated rats were given saline injections during the 8-day period.
STUDY 2: Reinstatement
The experiments consisted of 3 phases: training for alcohol self-administration, extinction of the drug-reinforced behavior and tests for reinstatement.
Training
Operant self-administration of alcohol was initiated on a FR-1 schedule for 60 min/day for 5–7 days per week. Responding on the FR-1 schedule was maintained for 8–13 sessions. The requirement for alcohol delivery was then increased to a FR-2 schedule for 4–5 sessions. Subsequently, the schedule requirement was increased to a FR-3 schedule for 8–19 sessions until the rats obtained 3 days of stable drug taking (less than 20% deviation from the mean).
Extinction
The conditions during the extinction sessions were identical to the training condition, with the exception that presses on the active lever did not result in the delivery of alcohol. Extinction sessions continued for 4–9 daily sessions until the rats reached the extinction criterion of less then 15 presses on the active lever during the 60-minute session. During the extinction phase, the rats were given daily injections of saline and were intubated daily with water (0.5 ml) in order to habituate them to the naltrexone/fluoxetine treatment procedure and to the procedure to deliver non-contingent alcohol priming.
Tests for Reinstatement
Experiment 1—Reinstatement: Naltrexone
Two groups of rats (n = 9 per group) were used. One group of rats was pretreated with saline, while the other group was pretreated with naltrexone (0.2 mg/kg, s.c.). Rats were initially tested for reinstatement after oral intubation of water (baseline condition). In the next three days, the rats were intubated, in a counterbalanced order, with 0.48, 0.72, and 0.96 mg/kg of alcohol (12 % w/v in tap water). The effects of pretreatment with saline or naltrexone on reinstatement induced by footshock (5 and 15 min; 0.8 mA, 0.5 sec ON; mean OFF period of 40 sec, range 10–70 sec) were then examined over the next two test days. The footshock was given in an ascending order. Throughout testing, saline and naltrexone were given 15 min before exposure to alcohol priming or footshock. Alcohol priming or footshock were given just prior to the start of the test sessions. The low dose of naltrexone, which was as effective as the high dose in decreasing alcohol self-administration in STUDY 1, was chosen for the reinstatement experiment.
Experiment 2—Reinstatement: Fluoxetine
Three groups of rats (n = 10 per group) were used. One group of rats was pretreated with saline and two other groups were pretreated with fluoxetine (2.5 or 5 mg/kg, i.p.). Rats were initially tested for reinstatement after oral intubation with water (baseline). During the next two days, the rats were tested for reinstatement after exposure to a priming dose of 0.48 mg/kg of alcohol (12 % w/v in tap water) and after exposure to footshock stress (15 min; 0.8 mA). The doses of fluoxetine are based on STUDY 1. The alcohol-priming dose and the duration of footshock are based on Experiment 1-Reinstatement.
Statistical Analyses
Study 1: Maintenance
As mentioned, two group of rats were used, saline and drug (naltrexone or fluoxetine) pretreatment. Within each drug pretreatment, the rats were exposed to the low dose of the drug (0.2 or 2.5 mg/kg for naltrexone and fluoxetine, respectively) for four days and then to the higher dose of the drug (0.4 or 5 mg/kg for naltrexone and fluoxetine, respectively) over the next four days. Therefore, three repeated measures ANOVAs were conducted for each drug. The first analysis compared the low dose of the drug with the saline pretreatment. The between-subject factor in this analysis was Drug Pretreatment (saline versus the low dose of naltrexone or fluoxetine) and the within-subject factor was Session (the four daily sessions). The second analysis was identical to the first one, but it included the high dose of naltrexone or fluoxetine. The third analysis compared, within each drug, the effect of Dose (low versus high).
Experiment 1—Reinstatement: Naltrexone
Two groups of rats were pretreated with either saline or naltrexone (0.2 mg/kg) and exposed to water priming, alcohol priming (0.24, 0.48, and 0.72 g/kg) and footshock (5 and 15 min) over six daily sessions. The water priming condition served as the baseline test against which to compare the effects of alcohol priming and footshock. Initially, an overall ANOVA of all six tests for reinstatement was done using the factor of Test Condition as the repeated-measures factor. Subsequently, two repeated-measures analyses were conducted, comparing the baseline condition versus alcohol priming or footshock. In the first analysis, the between-subject factor was Naltrexone Pretreatment (saline versus naltrexone) and the within-subject factor was Alcohol Priming (water—0 dose, 0.24, 0.48, and 0.72 g/kg). In the second analysis, the between-subject factor was Naltrexone Pretreatment and the within-subject factor was Footshock Duration (baseline— 0 min, 5, and 15 min).
Experiment 2-Reinstatement: Fluoxetine
Three groups of rats were pretreated with either saline, 2.5 or 5 mg/kg of fluoxetine and exposed to water priming, alcohol priming (0.48 g/kg) and footshock (15 min) over three daily sessions. The water priming condition served as the baseline test against which to compare the effects of alcohol priming and footshock. Initially, an overall ANOVA of all three tests for reinstatement was done using the factor of Test Condition as the repeated-measures factor. Subsequently, two repeated-measures analyses compared the baseline condition versus alcohol priming or footshock. In the first analysis, the between-subject factor was Fluoxetine Pretreatment (saline, 2.5 and 5 mg/kg) and the within-subject factor was Alcohol Priming (water versus 0.48 g/kg). In the second analysis, the between-subject factor was Fluoxetine Pretreatment and the within-subject factor was Footshock (baseline—0 min versus 15 min). In all analyses, significant differences were followed by Neuman-Kuels post hoc tests and the results are reported as statistically significant at a probability level of 5% or less.
RESULTS
STUDY 1: Maintenance
Experiment 1-Maintenance: Naltrexone
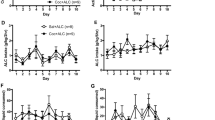
The mean intake of alcohol during the daily 30 min access to 12% alcohol solution was 1.5 ± 0.1 g/kg for the 14 rats selected for operant training. In the last four days, prior to naltrexone/saline pretreatment, the mean number of reinforcements earned was 18.3 ± 2.2 (an intake of 0.9 ± 0.05 g/kg). The effect of daily pretreatment with naltrexone on alcohol self-administration is shown in Figure 1. Naltrexone decreased oral alcohol self-administration, an effect that was stable over the eight days of testing and was maximal at the low dose (0.2 mg/kg). Analysis of variance for the low naltrexone dose showed a significant effect of Naltrexone Pretreatment (F[1,84] = 5.0, p < .05). No significant effects of Session (F[7,84] = .6, p > .05) or Naltrexone Pretreatment by Session (F[7,84] = .5, p > .05) were observed. Analysis of variance for the high naltrexone dose showed a significant effect of Naltrexone Pretreatment (F[1,36] = 5.3, p < .05). No significant effects of Session (F[7,84] = .6, p > .05) or Naltrexone Pretreatment by Session (F[7,84] = .4, p > .05) were observed. The statistical analyses within the naltrexone pretreatment group did not reveal an effect of Dose (F[1,18] = .2, p > .05).
Experiment 1—Maintenance: Naltrexone. Mean (± SEM) number of reinforcements earned in rats that self-administer alcohol on a FR-3 schedule of reinforcement. Rats were pretreated daily with naltrexone (0.2 mg or 0.4 mg/kg, s.c.; n = 7) or saline (n = 7) 15 min before the start of the self-administration session. * = Significant differences from the Naltrexone Group, p < .05.
Experiment 2—Maintenance: Fluoxetine
The mean intake of alcohol during the daily 30 min access to 12% alcohol solution was 0.9 ± 0.1 g/kg for the 16 rats selected for operant training. In the last four days, prior to fluoxetine/saline pretreatment, the mean number of reinforcements earned was 12.4 ± 1.4 (an intake of 0.67 ± 0.1 g/kg). The effect of daily pretreatment with fluoxetine on alcohol self-administration is shown in Figure 2. Fluoxetine decreased oral alcohol self-administration, an effect that was more pronounced at the high dose (5 mg/kg). Analysis of variance showed a significant effect of Fluoxetine Pretreatment (F[1,98] = 14.7, p < .01, and F[1,42] = 10.9, p < .01 for the low and high doses, respectively). No significant effects of Session or Fluoxetine Pretreatment by Session were observed (ps ⩾ .05). Within the fluoxetine pretreatment group, the Dose effect was also statistically significant (F[1,21] = 20.6, p < .01).
Experiment 2—Maintenance: Fluoxetine. Mean (± SEM) number of reinforcements earned in rats that self-administer alcohol on a FR-3 schedule of reinforcement. Rats were pretreated daily with fluoxetine (2.5 mg or 5 mg/kg, i.p.; n = 8) or saline (n = 8) 30 min before the start of the self-administration session. * = Significant differences from the Fluoxetine Group, p < .05.
STUDY 2: Reinstatement
Experiment 1-Reinstatement: Naltrexone
The mean intake of 12% alcohol for the 18 rats selected for operant training during the daily 30 min access to the drug in the ‘Richter’ tubes was 1.8 ± 0.6 g/kg. The mean number of responses on the active lever (reinforcers ± timeout responses) and on the inactive lever during the last four days of the FR-3 schedule were 66.1 ± 6.9 and 3.8 ± 0.7, respectively (mean intake of 1.2 ± 0.07 g/kg). During the first day of extinction, the mean numbers of responses on the previously active lever and on the inactive lever were 63.6 ± 7.3 and 3.8 ± 0.9, respectively. On the last day of extinction (i.e., the day in which individual rats obtained the extinction criterion [range 4–9 sessions]), the mean numbers of responses on the previously active lever and on the inactive lever were 8.6 ± 1.0 and 2.3 ± 0.6, respectively.
The effect of pretreatment with naltrexone (0.2 mg/kg) on reinstatement of alcohol seeking induced by priming injections of alcohol and exposure to footshock is shown in Figure 3. Analyses were conducted separately for the active and the inactive levers. Both alcohol priming and intermittent footshock reinstated alcohol seeking, as indicated by increased responding on the active lever. More important, naltrexone blocked the effect of alcohol priming, but did not alter reinstatement induced by a footshock stressor. Initial repeated measures ANOVA for responses on the active lever across the test conditions revealed a significant effect of Test Condition (F[5,107] = 4.5, p < .01). Subsequent repeated measures ANOVA, comparing water priming (baseline) with alcohol priming, revealed significant effects of Naltrexone Pretreatment (F[1,48] = 15.3, p < .01), Alcohol Priming (F[3,48] = 5.9, p < .01), and Naltrexone Pretreatment by Alcohol Priming (F[3,71] = 2.7, p < .05). No significant effects were observed for the analysis of responses on the inactive lever (ps ⩾ .05). Repeated-measures ANOVA, comparing the baseline condition with footshock, revealed significant effects of Footshock (F[2,30] = 6.8, p < .01), but not of Naltrexone Pretreatment (F[1,30] = 0.2, ns). No significant effects were observed for the analysis of responses on the inactive lever (ps ⩾ .05).
Experiment 1—Reinstatement: Naltrexone. Mean (± SEM) number of responses on the active lever after water priming (baseline condition), alcohol priming (0.48, 0.72, and 0.96 g/kg) and exposure to intermittent footshock stress (5 and 15 min, 0.8 mA; 0.5 sec ON; mean OFF period of 40 sec, range 10–70 sec). Rats were pretreated with naltrexone (0.2 mg/kg, s.c., n = 9) or saline (n = 9) 15 min before exposure to water priming, alcohol priming or footshock. 1 = Significant differences from the Naltrexone condition, p < .05. 2 = Significant differences from the Baseline, water priming condition, p < .05.
Experiment 2—Reinstatement: Fluoxetine
The mean intake of 12% alcohol by the 30 rats selected for operant training during the daily 30 min access to the drug in the “Richter” tubes was 1.18 ± 0.05 g/kg. The mean number of responses on the active lever (reinforcers ± timeout responses) and on the inactive lever during the last four days of the FR-3 schedule was 68.4 ± 5.4 and 5.4 ± 0.5, respectively (mean intake of 1.01 ± 0.08 g/kg). During the first day of extinction, the mean number of responses on the previously active lever and on the inactive lever was 50.6 ± 4.1 and 6.5 ± 1.6, respectively. On the last day of extinction, the mean number of responses on the previously active lever and on the inactive lever was 11.7 ± 1.7 and 3.9 ± 0.9, respectively.
The effect of pretreatment with fluoxetine (2.5 and 5 mg/kg) on reinstatement of alcohol seeking induced by priming injections of alcohol and exposure to footshock is shown in Figure 4.. Analyses were conducted separately for the active and the inactive levers. Both alcohol priming and intermittent footshock reinstated alcohol seeking. More important, fluoxetine attenuated the effect of footshock on reinstatement, while its effect on reinstatement induced by alcohol priming was less consistent.
Experiment 2—Reinstatement: Fluoxetine. Mean (± SEM) number of responses on the active lever after water priming (baseline condition), alcohol priming (0.48 g/kg) and exposure to intermittent footshock stress (15 min, 0.8 mA). Rats were pretreated with fluoxetine (2.5 and 5 mg/kg, i.p., n = 10 per dose) or saline (n = 10) 30 min before exposure to water priming, alcohol priming or footshock. 1 = Significant differences from the Fluoxetine condition, p < .05. 2 = Significant differences from the Baseline, water priming condition, p < .05.
Initial repeated measures ANOVA for responses on the active lever across the test conditions revealed a significant effect of Test Condition (F[2,89] = 8.6, p < .01). Subsequent repeated measures ANOVA, comparing water priming (baseline) with alcohol priming, revealed a significant effect of Alcohol Priming (F[1,27] = 14.5, p < .01). The effect of Fluoxetine Pretreatment was not statistically significant (F[2,27] = .1, p > .05). No significant effects were observed for the analysis of responses on the inactive lever (ps ⩾ .05). Repeated measures ANOVA, comparing the baseline condition with footshock, revealed significant effects of Footshock (F[1,27] = 8.2, p < .01), Fluoxetine Pretreatment (F[2,27] = 8.6, p < .01) and Footshock by Fluoxetine Pretreatment (F[1,27] = 9.8, p < .01). No significant effects were observed for the analysis of responses on the inactive lever (ps ⩽ .05).
DISCUSSION
In this series of studies we tested the effects of fluoxetine and naltrexone on alcohol self-administration and reinstatement of alcohol seeking induced by drug priming and exposure to intermittent footshock. We have used an oral alcohol self-administration procedure in which rats, with free access to food and water in their home cage, self-administer the drug during the 60-minute daily sessions. While the intake of alcohol was variable across the experiments, the amounts of the drug consumed exceed levels of intake that have been shown to produce reliable pharmacological effects (cf. Linseman 1987; Weiss et al. 1990). In STUDY 1, it was found that during the maintenance phase, low doses of naltrexone and fluoxetine decrease lever presses for alcohol. These data are in agreement with previous reports which have shown that preferentially mu opioid receptor antagonists and SSRI agents, at doses similar to the ones used in the present report, decrease alcohol self-administration during the maintenance phase (e.g., Murphy et al. 1988: Haraguchi et al. 1990; Hyytia and Sinclair 1993; Schwarz-Stevens et al. 1992; see Amit et al. 1991 and Ulm et al. 1995 for reviews).
In STUDY 2, the effects of fluoxetine and naltrexone on reinstatement of alcohol seeking induced by alcohol priming and footshock stress were determined. The doses used in the reinstatement experiments were based on STUDY 1. As in our previous report (Lê et al. 1998), footshock was found to be more effective than alcohol priming in reinstating drug seeking. The reasons for the potent effect of the footshock stressor on reinstatement of alcohol seeking as compared to alcohol priming are not known. It should be pointed out, however, that this observation is not unique to reinstatement of alcohol seeking. In heroin-trained rats, rate of responding during tests for reinstatement after intermittent footshock (10–60 min) was higher than after priming injections of heroin (0.125–0.5 mg/kg, s.c.) (Shaham 1996; Shaham et al. 1996).
One of the main findings of this report is that naltrexone (0.2 mg/kg) blocked alcohol-induced reinstatement, but had no effect on reinstatement induced by footshock. These data, though based on only one low dose, suggest that, as in the case of heroin priming (Shaham and Stewart 1996; Stewart 1984), activation of opioid receptors is critical for alcohol-induced reinstatement. The brain mechanisms involved in alcohol-induced reinstatement, however, are not known. Stewart (1984) has shown that activation of opioid receptors in the ventral tegmental area, the cell body region of the mesolimbic dopamine (DA) system, contributes to reinstatement induced by priming injections of heroin. We speculate that alcohol priming might also induce reinstatement by activating the mesolimbic DA system. This speculation is based on the observation that naltrexone blocks reinstatement induced by both heroin priming and alcohol priming. In addition, it has been shown that naltrexone blocked alcohol-induced DA release in the terminal region of the nucleus accumbens (Gonzales and Weiss 1998).
The same dose of naltrexone that blocked alcohol-induced reinstatement, however, did not alter footshock-induced reinstatement. It is possible that higher doses of naltrexone might have been effective against footshock. This possibility, however, is unlikely because higher doses of naltrexone (1–10 mg/kg) did not alter stress-induced reinstatement in heroin-trained rats (Shaham and Stewart 1996). Taken together, it appears that activation of the endogenous opioid system by footshock (see Akil et al. 1976; Amit and Galina 1986) is not involved in reinstatement induced by footshock stress.
Another main finding in this report is that fluoxetine blocked stress-induced reinstatement, while its effect on alcohol-induced reinstatement was less consistent. Interpretation of these latter data, however, is not straightforward. Specifically, there were no differences between the groups pretreated with vehicle or fluoxetine and exposed to alcohol priming. On the other hand, within each group, as compared with water priming, the priming effect of alcohol was statistically significant in rats pretreated with the vehicle, but not in rats pretreated with fluoxetine.
The reasons for the profound effect of fluoxetine on stress-induced reinstatement are not known. One possibility is that fluoxetine decreases the impact of footshock due to its analgesic effect. Fluoxetine has been shown to decrease sensitivity to footshock (Messing et al. 1975) and to potentiate footshock-induced analgesia (Tricklebank et al. 1982). In other studies, however, acute or repeated injections of fluoxetine, at doses higher than the ones used in the present study (10 mg/kg), did not alter sensitivity to footshock (Akunne and Soliman 1994; Nelson et al. 1997). In addition, we found that fluoxetine (5 mg/kg) did not alter sensitivity to footshock as compared with saline (data not shown). Shock sensitivity was measured by determining the threshold intensity for inducing the withdrawal of the hind paw from the grid floor after exposure to footshock. Taken together, it is unlikely that fluoxetine decreased footshock-induced reinstatement by attenuating the nociceptive effect of footshock.
An additional possibility is that fluoxetine decreases stress-induced reinstatement by reducing anxiogenic responses induced by footshock. It is important to note, however, that the available data can neither refute nor support this idea. Several studies found that fluoxetine decreased anxiogenic responses in animal models of anxiety (e.g., Abe et al. 1998). A number of other studies, however, failed to demonstrate these effects (e.g., De Vry et al. 1993; Griebel et al. 1997), which appear to a large degree model-dependent (Sanchez and Meier 1997). In addition, several studies showed that the anxiolytic effects of fluoxetine only emerge after several weeks of drug administration (Griebel et al. 1995; Bodnoff et al. 1989). These anxiolytic actions of chronic fluoxetine treatment cannot account for the present data because the rats were only given fluoxetine during the 3-daily tests. Finally, in studies using the reinstatement procedure, it was found that corticotropin releasing factor (CRF) receptor antagonists or alpha-2 adrenoceptor agonists (drugs that show anxiolytic-like effects in several studies) decrease footshock-induced reinstatement of drug seeking (Erb et al. 1998a, b; Shaham et al. 1997, 1998a). On the other hand, attempts to mimic the effect of footshock on reinstatement of heroin seeking with anxiogenic compounds such as FG-7142 or PTZ were not successful (Y. Shaham, unpublished data). Taken together, with the available data it cannot be unambiguously concluded that fluoxetine blocks stress-induced reinstatement by decreasing the anxiogenic effects of footshock.
The observation that fluoxetine attenuates footshock-induced reinstatement is not readily predicted from previous reports on the neurochemical effects of this drug. Specifically, there exist several neurochemical similarities between the effects induced by SSRI agents and stressors. Fluoxetine and other SSRI agents increase extracellular levels of serotonin in terminal regions of the serotonergic system (Fuller 1994). Similarly, many studies using post-mortem tissue assays and microdialysis indicate that footshock and other stressors increase the release of serotonin in terminal regions (see Bliss et al. 1972; Rueter et al. 1997). In addition, SSRI agents, and other manipulations that increase serotonin neurotransmission, activate the hypothalamic-pituitary-adrenal (HPA) axis and the autonomic nervous system (Chaouloff 1993; Fuller and Snoody 1990), known to be activated during stress (Cannon 1935; Selye 1956). It should be noted, however, that it is unlikely that activation of the HPA axis is involved in stress-induced reinstatement. Footshock-induced reinstatement in heroin- and cocaine-trained rats remains intact in adrenalectomized heroin-trained rats or in adrenalectomized cocaine-trained rats that were given corticosterone replacement via pellets (Erb et al. 1998b; Shaham et al. 1997). As for stress and activation of the serotonin system, recent reports by Lucki and colleagues demonstrate that, at least in the case of swim stress, there are profound regional differences in the effect of stress on extracellular levels of serotonin. The swim stress has been shown to increase serotonin levels in the striatum by about 90%, but to decrease it in the amygdala and the septal area by about 40-50% (Kirby et al. 1995; Kirby and Lucki 1997). These latter findings may be relevant to the understanding of relapse to drug seeking induced by stressors. The amygdala is one of the main brain areas involved in the stress response (Aggleton 1992) and, therefore, might also be involved in stress-induced reinstatement. As for the septum, we have recently found that reversible inactivation of the septum with tetrodotoxin has similar effects to those of footshock on reinstatement of heroin seeking (Shaham et al. 1998b).
It should be noted, however, that although we interpret the data with fluoxetine to indicate that serotonin is involved in stress-induced reinstatement, other possibilities should be considered. Previous work on the neuronal mechanisms involved in the effect of fluoxetine and other SSRI agents on alcohol consumption, activation of the HPA axis and suppression of feeding behavior do not provide clear evidence that these effects are directly related to the action of these compounds on the serotonergic system. Specifically, the effects of SSRI agents on the HPA axis, alcohol consumption and feeding were not altered by serotonin receptor antagonists or by neurotoxic lesions of the serotonergic system (Amit et al. 1991; Fuller and Snoody 1990; Grignaschi and Samanin 1992). Therefore, it is possible that the effect of fluoxetine on footshock-induced reinstatement is mediated by a non-serotonergic mechanism that is yet to be identified.
We have found that naltrexone blocked alcohol-induced, but not stress-induced reinstatement. In contrast, fluoxetine blocked stress-induced reinstatement, while its effect on alcohol-induced reinstatement was less consistent. We interpret these data to indicate that different neurochemical substrates are involved in reinstatement induced by alcohol priming and footshock. An alternative explanation, however, is that these effects of fluoxetine and naltrexone during tests for reinstatement are due to their differential effect on high (in the case of footshock-induced reinstatement) versus low (in the case of alcohol-induced reinstatement) response rates. Many studies have shown that the behavioral actions of drugs depend on rate of responding (see Sanger and Blackman 1976). It appears, however, that a rate-dependent hypothesis cannot account for the present data. To the best of our knowledge, fluoxetine and naltrexone do not have different effects on high versus low response rates. In addition, in Study 1 (Maintenance), the drugs had similar effect on rate of responding for alcohol. It should be pointed out that rate of responding for alcohol during maintenance under the FR-3 schedule (about 40–70 responses on the active lever/1 hr) is similar to that observed after exposure to footshock during tests for reinstatement.
In conclusion, regardless of the exact mechanism, in this study we found that naltrexone was effective in blocking reinstatement induced by alcohol priming, while fluoxetine was effective in blocking reinstatement induced by exposure to stress. The effect of naltrexone on reinstatement of drug seeking induced by alcohol priming is consistent with findings obtained in humans. In these studies, naltrexone reduced relapse rates in subjects that sampled alcohol during a drug-free period (O'Malley et al. 1992; Volpicelli et al. 1992). The correspondence between the present data with fluoxetine and studies with humans with this drug is less clear. Fluoxetine has a modest effect on relapse to alcohol use in humans (Naranjo and Sellers 1989; Sellers et al. 1992). Our data suggest that to the extent that the reinstatement procedure models relapse to drug use in humans, fluoxetine might be more effective in the treatment of people that relapse to alcohol as a result of exposure to aversive life events or stressors. Finally, because multiple factors contribute to relapse to drugs, the present data suggest that a pharmacological therapy that combines naltrexone and fluoxetine might be more effective in relapse prevention than either therapy alone.
References
Abe M, Nakai H, Tabata R, Saito K, Egawa M . (1998): Effect of 5-[3-[((2S)-1,4-benzodioxan-2-ylmethyl)amino]propoxy]-1,3-benzodioxole HCl (MKC-242), a novel 5-HT1A-receptor agonist, on aggressive behavior and marble burying behavior in mice. Jpn J Phrmacol 76: 297–304
Aggleton JP . (1992): The amygdala: neurobiological aspects of emotion, memory, and mental dysfunction. New York, Wiley-Liss
Akil H, Madden J, Patrick RL, Barchas JD . (1976): Stress-induced increase in endogenous opiate peptides: Concurrent analgesia and its partial reversal by naloxone. In Kosterlits HW (ed), Opiates and Endogenous Opioid Peptides. Amsterdam, The Netherlands, Elsevier, pp 63–70
Akunne HC, Soliman KF . (1994): Serotonin modulation of pain responsiveness in the aged rat. Pharmacol Biochem Behav 48: 411–416
Amit Z, Galina H . (1986): Stress-induced analgesia: Adaptive pain suppression. Physiol Rev 66: 1091–1120
Amit Z, Smith BR, Gill K . (1991): Serotonin uptake inhibitors: Effects on motivated consummatory behaviors. J Clin Psychiatry 52(suppl):55–60
Bigelow GE, Griffiths RR, Liebson IA . (1977): Pharmacological influences upon human ethanol self-administration. In: Gross MM (ed), Alcohol intoxication and withdrawal. New York, Plenum Press, pp 523–538
Bliss EL, Thatcher W, Ailion J . (1972): Relationship of stress to brain and serotonin and 5-hydroxyindoleacetic acid. J Psychiatry Res 9: 71–80
Bodnoff SR, Suranyi-Cadotte B, Quirion R, Meaney MJ . (1989): A comparison of the effects of diazepam versus several typical and atypical anti-depressant drugs in an animal model of anxiety. Psychopharmacology 97: 277–279
Brown SA, Vik PW, Patterson TL, Grant I, Schuckit MA . (1995): Stress, vulnerability and adult alcohol relapse. J Stud Alcohol 56: 538–545
Cannon WB . (1935): Stresses and strains of homeostasis. Am J Med Sci 189: 1–14
Carroll ME, Comer SD . (1996): Animal models of relapse. Exp Clin Psychopharmacol 4: 11–18
Chaouloff F . (1993): Physiopharamcological interactions between stress hormones and central serotonergic systems. Brain Res Rev 18: 1–32
Cooper ML, Russell M, Skinner JB, Frone MR, Mudar P . (1992): Stress and alcohol use: Moderating effects of gender, coping and alcohol expectancies. J Abnorm Psychol 101: 139–152
de Wit H . (1996): Priming effects with drugs and other reinforcers. Exp Clin Psychopharmacol 4: 5–10
de Wit H, Chutuape MA . (1993): Increased ethanol choice in social drinkers following ethanol preload. Behav Pharmacol 4: 29–36
De Vry J, Benz U, Schreiber R, Traber J . (1993): Shock-induced ultrasonic vocalization in young adult rats: A model for testing putative anti-anxiety drugs. Eur J Pharmacol 16: 331–339
Erb S, Shaham Y, Stewart J . (1996): Stress reinstates cocaine-seeking behavior after prolonged extinction and drug-free periods. Psychopharmacology 128: 408–412
Erb S, Mueller D, Shaham Y, Leung S, Stewart J . (1998a): Effect of clonidine on stress- and drug induced relapse to heroin and cocaine seeking. Soc Neurosci Abstr 24: 498
Erb S, Shaham Y, Stewart J . (1998b): The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. J Neurosci 14: 5529–5536
Fuller RW . (1994): Uptake inhibitors increase extracellular serotonin concentration measured by brain microdialysis. Life Sci 55: 163–167
Fuller RW, Snoody HD . (1990): Serotonin receptors subtypes involved in the elevation of serum corticosterone concentration in rats by direct- and indirect serotonin agonists. Neuroendocrinology 52: 206–211
Gonzales RA, Weiss F . (1998): Suppression of ethanol-reinforced behavior by naltrexone is associated with atenuation of ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J Neurosci 18: 10663–10671
Grignaschi G, Samanin R . (1992): Role of serotonin and catecholaines in brain in the feeding suppressant effect of fluoxetine. Neuropharmacology 31: 445–449
Griebel G, Blanchard DC, Agnes RS, Blanchard RJ . (1995): Differential modulation of antipredator defensive behavior in Swiss-Webster mice following acute and chronic administration of imipramine and fluoxetine. Psychopharmacology 120: 57–66
Griebel G, Rodgers RJ, Perrault G, Sanger DJ . (1997): Risk assessment behaviour: evaluation of utility in the study of 5-HT-related drugs in the rat elevated plus-maze test. Pharmacol Biochem Behav 57: 817–827
Haraguchi M, Samson HH, Tolliver GA . (1990): Reduction in oral ethanol self-administration in the rat by the 5-HT uptake blocker fluoxetine. Pharmacol Biochem Behav 35: 259–262
Herz A . (1997): Endogenous opioid systems and alcohol addiction. Psychopharmacology 129: 99–111
Hodgson R, Rankin H, Strickwell T . (1979): Alcohol dependence and the priming effect. Behav Res Ther 17: 379–387
Hore BD . (1971): Factors in alcoholic relapse. Brit J Addict 66: 89–96
Hyytia P, Sinclair JD . (1993): Responding for oral ethanol after naltrexone treatment by alcohol-preferring AA rats. Alcoholis: Clin Exp Res 17: 631–636
Kirby LG, Allen AR, Lucki I . (1995): Regional differences in the effects of forced swimming on extracellular levels of 5-hydroxytryptamine and 5-hydroxyindoleacetic acid. Brain Res 682: 189–196
Kirby LG, Lucki I . (1997): Interaction between the forced swimming test and fluoxetine treatment on extracellular 5-hydroxytryptamine and 5-hydroxyindoleacetic acid in the rat. J Pharmacol Exp Ther 282: 967–976
Lê AD, Tomkins DM, Sellers EM . (1996): Uses of serotonin and opiate based drugs in the pharmacotherapy of alcohol dependence: An overview of the preclinical data. Alcohol Alcoholism 31: 27–32
Lê AD, Quan B, Juzystch W, Fletcher PJ, Joharchi N, Shaham Y . (1998): Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology 135: 169–174
Linseman MA . (1987): Alcohol consumption in free feeding rats: Procedure, genetic and pharmacokinetic factors. Psychopharmacology 92: 254–261
Ludwig AM, Wikler A, Stark LH . (1974): The first drink. Psychobiological aspects of craving. Arch Gen Psychiatry 30: 539–547
Messing RB, Phebus L, Fisher LA, Lytle LD . (1975): Analgesic effect of fluoxetine hydrochloride (Lilly 110140), a specific inhibitor of serotonin uptake. Psychopharmacol Commun 1: 511–521
Murphy JM, Waller MB, Gatto GJ, McBride WJ, Lumeng L, Li TK . (1988): Effects of fluoxetine on the intragastric self-administration of ethanol in the alcohol preferring P line of rats. Alcohol 5: 283–286
Naranjo CA, Sellers EM . (1989): Serotonin uptake inhibitors attenuate ethanol intake in problem drinkers. Recent Dev Alcohol 7: 255–266
Nelson CJ, Jordan WP, Bohan RT . (1997): Daily fluoxetine administration impairs avoidance learning in the rat without altering sensory thresholds. Prog Neuropsychopharmacol Biol Psychiatry 21: 1043–1057
O'Malley SS, Jaffe JH, Chang G, Schottenfeld RS, Meyer RE, Rounsaville BJ . (1992): Naltrexone and coping skills therapy for alcohol dependence: A controlled study. Arch Gen Psychiatry 49: 881–887
Rueter LE, Fornal CA, Jacobs BL . (1997): A critical review of 5-HT brain microdialysis and behavior. Rev Neurosci 8: 117–137
Sanchez C, Meier E . (1997): Behavioral profiles of SSRIs in animal models of depression, anxiety and aggression. Are they all alike? Psychopharmacology 129: 197–205
Sanger DJ, Blackman DE . (1976): Rate-dependent effects of drugs: A review of the literature. Pharmacol Biochem Behav 4: 73–83
Schwarz-Stevens KS, Files FJ, Samson HH . (1992): Effects of morphine and naloxone on ethanol- and sucrose-reinforced responding in non-deprived rats. Alcoholism: Clin Exp Res 16: 822–832
Sellers EM, Higgins GA, Sobell MB . (1992): 5-HT and alcohol abuse. Trends Pharmacol Sci 13: 69–75
Selye H . (1956): The stress of life. New York, McGraw-Hill
Shaham Y . (1996): Effect of stress on opioid-seeking behavior: Evidence from studies with rats. Annal Behav Med 18: 255–263
Shaham Y, Stewart J . (1995): Stress reinstates heroin self-administration behavior in drug-free animals: An effect mimicking heroin, not withdrawal. Psychopharmacology 119: 334–341
Shaham Y, Stewart J . (1996): Effects of opioid and dopamine receptor anatgonist on relapse induced by stress and re-exposure to heroin in rats. Psychopharmacology 125: 385–391
Shaham Y, Rajabi H, Stewart J . (1996): Relapse to heroin-seeking under opioid maintenance: the effects of opioid withdrawal, heroin priming and stress. J Neurosci 16: 1957–1963
Shaham Y, Funk D, Erb S, Brown TJ, Walker CD, Stewart J . (1997): Corticotropin-releasing factor, but not cortico sterone, is involved in stress-induced relapse to heroin-seeking in rats. J Neurosci 17: 2605–2614
Shaham Y, Erb S, Leung S, Buczek Y, Stewart J . (1998a): CP-154,526, a selective, non peptide antagonist of the corticotropin-releasing factor type 1 receptor attenuates stress-induced relapse to drug seeking in cocaine-and heroin-trained rats. Psychopharmacology 137: 184–190
Shaham Y, Leung S, McDonald RJ, Stewart J . (1998b): Disruption of inhibitory processes may be involved in stress-induced relapse to heroin. Soc Neurosci Abstr 24: 498
Sinclair JD, Senter RJ . (1967): Increased preference for ethanol in rats following alcohol deprivation. Psychonom Sci 8: 11–16
Stewart J . (1984): Reinstatement of heroin and cocaine self-administration behavior in the rat by intracerebral application of morphine in the ventral tegmental area. Pharmacol Biochem Behav 20: 917–923
Stewart J, de Wit H . (1987): Reinstatement of drug-taking behavior as a method of assessing incentive motivational properties of drugs. In Bozarth MA (ed), Methods of assessing the reinforcing properties of abused drugs. Springer-Verlag, New York, pp 211–227
Tricklebank MD, Hutson PH, Curzon G . (1982): Analgesia induced by brief footshock is inhibited by 5-hydroxytryptamine but unaffected by antagonists of 5-hydroxytryptamine or by naloxone. Neuropharmacology 21: 51–56
Ulm RR, Volpicelli JR, Volpicelli LA . (1995): Opiates and alcohol self-administration in animals. J Clin Psychiatry 56(suppl 7):5–14
Volpicelli JR, Anterman AI, Hayashida M, O'Brien CP . (1992): Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry 49: 876–880
Volpicelli JR, Rhines KC, Rhines JS, Volpicelli LA, Alterman AI, O'Brien CP . (1997): Naltrexone and alcohol dependence: role of subject compliance. Arch Gen Psychiatry 54: 737–742
Weiss F, Mitchiner M, Bloom FE, Koob GF . (1990): Free-choice responding for ethanol versus water in alcohol prefering (P) and unselected Wistar rats is differentially modified by naloxone, bromocriptine and methysergide. Psychopharmacology 101: 178–186
Zernig G, Fabisch K, Fabisch H . (1997): Pharmacotherapy for alcohol dependence. Trends Pharmacol Sci 18: 229–231
Acknowledgements
This study was supported by grants from the Addiction Research Foundation (AD Lê) and the MRC of Canada (YS).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lê, A., Poulos, C., Harding, S. et al. Effects of Naltrexone and Fluoxetine on Alcohol Self-Administration and Reinstatement of Alcohol Seeking Induced by Priming Injections of Alcohol and Exposure to Stress. Neuropsychopharmacol 21, 435–444 (1999). https://doi.org/10.1016/S0893-133X(99)00024-X
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1016/S0893-133X(99)00024-X
Keywords
This article is cited by
-
Blocking μ-opioid receptors attenuates reinstatement of responding to an alcohol-predictive conditioned stimulus through actions in the ventral hippocampus
Neuropsychopharmacology (2023)
-
Learning processes in relapse to alcohol use: lessons from animal models
Psychopharmacology (2023)
-
Reinstatement of Pavlovian responses to alcohol cues by stress
Psychopharmacology (2023)
-
CRF-5-HT interactions in the dorsal raphe nucleus and motivation for stress-induced opioid reinstatement
Psychopharmacology (2021)
-
Naloxone effects on extinction of ethanol- and cocaine-induced conditioned place preference in mice
Psychopharmacology (2017)