Abstract
The extended amygdala is composed of the central and medial amygdaloid nucleus which through the sublenticular extended amygdala (SLEA) and the interstitial nucleus of the posterior limb of the anterior commissure (IPAC) merge into the bed nucleus of stria terminals (BST). Based on anatomical connections with limbic areas, the extended amygdala has been proposed to play an important role in cognitive and affective processes. This study examines the effect of the atypical antipsychotic clozapine and the classical antipsychotic haloperidol on Fos-like-immunoreactivity (FLI) induction in areas belonging to the extended amygdala. Acute administration of clozapine (10–20 mg/kg) induced FLI in the central amygdaloid nucleus, IPAC, SLEA, and BST lateral division and, as previously described, in areas connected to the extended amygdala, such as the prefrontal cortex and nucleus accumbens shell. In contrast, acute administration of haloperidol (0.1–1 mg/kg) failed to induce FLI in the BST lateral division and SLEA but increased FLI in the IPAC. A small increase in FLI was observed in the central amygdaloid nucleus after 0.1 but not after 1 mg/kg of haloperidol. The present results, showing a preferential influence of clozapine, as compared to haloperidol, in the extended amygdala propose a new brain structure involved in the pharmacological effects of atypical antipsychotics.
Similar content being viewed by others
Main
The amygdaloid complex is an ensemble of nuclei that play a central role in emotional processes, particularly in the assignment of affective significance to specific stimuli (Aggleton 1993; Aggleton and Mishkin 1985; Gallagher and Chiba 1996; Le Doux 1995). Information elaborated in the basolateral amygdaloid nuclei enter into the central amygdaloid nucleus, which conveys the stimuli together to elicit appropriate behavioral responses (Pitkänen et al. 1997).
Neuronal cell groups composing the central and medial amygdaloid nucleus form a continuum of cells that extend through the sublenticular extended amygdala (SLEA) and the interstitial nucleus of the posterior limb of the anterior commissure (IPAC) to merge with the bed nucleus of stria terminalis (BST) and form the so-called extended amygdala (Alheid and Heimer 1988; Alheid et al. 1995; Heimer et al. 1997b).
The central division of the extended amygdala, which is formed by the central amygdaloid nucleus, BST lateral division, IPAC, and SLEA central division, establishes reciprocal neuronal connections with the caudal aspect of the nucleus accumbens shell (Acb shell) and is also connected with the prefrontal cortex (de Olmos et al. 1985; Heimer et al. 1997a; Heimer et al. 1997b; Zahm and Brog 1992).
Based on anatomical connections, the extended amygdala seems to be an important site involved in neuropsychiatric disorders (Heimer et al. 1997b); however, specific data showing the effect of drugs used in the therapy of these disorders in the extended amygdala are scarce. Induction of the early gene c-fos by antipsychotics in the central amygdaloid nucleus (Sebens et al. 1995) and antagonism of the excitatory effects induced by a local infusion of amphetamine in the amygdaloid complex by clozapine (Wang and Rebec 1996) have been reported. Moreover, it was recently shown (Beck 1994; Duncan et al. 1996; Morelli et al. 1999) that antidepressant drugs inhibiting either noradrenaline or serotonin re-uptake, induced the expression of the early gene c-fos in areas belonging to the rat extended amygdala, suggesting a role of the extended amygdala in disorders characterized by affective disturbance.
Induction of the early gene c-fos is secondary to neuronal activation and is correlated to an increased function of specific areas in the CNS (Sagar et al. 1988). Moreover, detection of the c-fos-encoded protein Fos, through fos-like-immunoreactivity (FLI), allows the study of the influence of drugs in small brain areas, such as those examined in this study, and analysis of several brain areas at the same time and in the same experimental animal.
Using this methodology, it has been shown that classical and atypical antipsychotics, such as haloperidol and clozapine, induced the same pattern of FLI in the nucleus accumbens and lateral septum, whereas, they induced a different pattern of FLI in the striatum and prefrontal cortex, which is predictive of their ability to cause extrapyramidal side effects and to be effective on the negative symptoms of schizophrenia (Deutch and Duman 1996; Fibiger 1994; MacGibbon et al. 1994; Merchant et al. 1996; Nguyen et al. 1992; Robertson and Fibiger 1992; Robertson et al. 1994; Wan et al. 1995).
To evaluate the potential role of the extended amygdala in the effects of antipsychotic drugs, particularly those effective in cases that respond poorly or not at all to classic antipsychotics, we evaluated the induction of c-fos through FLI in areas of the rat brain belonging to the extended amygdala after acute administration of the atypical antipsychotic clozapine, which reduces the negative symptoms of schizophrenia, and haloperidol, which belongs to classical antipsychotics and which, differently from clozapine, induces extrapyramidal side effects (Brunello et al. 1995; Kane et al. 1988).
METHODS
Experimental Protocol
Male Sprague–Dawley rats (200–250 g) were used in all the experiments. Different groups of rats received one of the following treatments: vehicle (n = 6), clozapine (10 mg/kg, n = 6), (20 mg/kg, n = 7), haloperidol HCl (0.1 mg/kg, n = 5) (1 mg/kg, n = 4). Clozapine was dissolved in slightly acidified (pH 6.0) saline and injected IP, haloperidol was diluted with saline from SERENASE (Lusofarmaco, Italy) and injected SC.
To minimize the influence of stress on the induction of FLI in limbic areas, rats were habituated to the manipulation that precedes the injection during 4 days before the experiment. Rats were also kept in the animal room and in a familiar cage during the experiment.
Fos Immunohistochemistry
The different groups of rats were anesthetized with chloralhydrate 120 min after vehicle, clozapine, or haloperidol administration. Rats were then perfused transcardially with saline followed by 4% paraformaldehyde dissolved in 0.1 M sodium phosphate buffer, pH 7.4, and their brains, postfixed in the same solution, were cut coronally on a vibratome (40 μm) 2 days later. Sections were incubated for 48 h with a fos primary antibody selected from a conserved region of mouse and human c-fos (OA-11-824, Cambridge Research Biochemical) at a dilution of 1:1,400. The reaction was visualized using biotinylated secondary antisera and by standard avidin-biotin-horseradish peroxidase technique. Control sections were incubated in the presence of the fos peptide.
Fos-immunoreactivity was quantified with an image analyzer (IBAS, Zeiss) by counting the number of Fos-like positive nuclei. We considered as Fos-like positive only neurons showing gray levels ranging between 0 and 110/120 (total range was from 0 to 255) in order to eliminate FLI background as well as very light stained nuclei constitutively present all over the brain. The number of stained cells in each structure was counted in the two brain sides of each rat, and the values were averaged for each experimental group.
Drugs
Clozapine was kindly donated by Polfa (Starogard, Poland), haloperidol (SERENASE, Lusofarmaco, Italy) was purchased from commercial sources. Drugs were injected in a volume of 0.3 ml IP or 0.1 ml SC/100 g body weight.
Statistics
Mean and SEM were calculated. Significance between groups was evaluated by analysis of variance (ANOVA) followed by Student–Newman-Keuls test.
RESULTS
Figure 1 shows the levels in which the number of fos-like positive nuclei were determined. Section levels were obtained from the Atlas of Paxinos and Watson (1986).
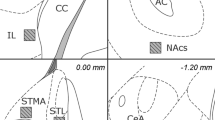
Drawing of representative sections used for fos-like positive neurons counting. A: prefrontal cortex (PFCx); B: nucleus accumbens shell (Sh), core (Co), dorsolateral striatum (CPu); C: bed nucleus of stria terminalis lateral division (BSTL); D: interstitial nucleus of the posterior limb of the anterior commissure (IPAC), sublenticular extended amygdala (SLEA); E: central amygdaloid nucleus (CE). Sections were taken from the Atlas of Paxinos and Watson (1986).
Fos-Like-Immunoreactivity After Clozapine
Acute administration of clozapine at either 10 or 20 mg/kg IP induced an increase in FLI in the prefrontal cortex, Acb shell, BST lateral division, and central amygdaloid nucleus (Table 1, Figures 2 and 3 ). Lower increase in FLI was observed in the Acb core, dorsolateral striatum, IPAC and SLEA (Table 1, Figures 2 and 3).
Number of fos-like positive nuclei in: bed nucleus of stria terminalis lateral division (BSTL), interstitial nucleus of the posterior limb of the anterior commissure (IPAC), sublenticular extended amygdala (SLEA), central amygdaloid nucleus (Amyg Ce) after vehicle or clozapine. ** p < .001 vs. vehicle.
Photomicrographs of a coronal section showing fos-like positive nuclei in: A, B, C, bed nucleus of stria terminalis lateral division (BSTL), D, E, F, interstitial nucleus of the posterior limb of the anterior commissure (IPAC), G, H, I, central amygdaloid nucleus (Ce) after vehicle (A, D, G), 1 mg/kg SC of haloperidol (B, E, H), or 20 mg/kg IP of clozapine (C,F,I). CPu = striatum, ac = anterior commissure, GP = globus pallidus, ic = internal capsule. Scale bar = 0.5 mm.
The small number of fos-like positive nuclei observed in IPAC and SLEA was attributable to the anatomical characteristics of these two portions of the central extended amygdala, which form two corridors of sparse cells connecting the central amygdaloid nucleus to the BST lateral division (Figure 4 ). Therefore, coronal sections such as those used for FLI quantification do not allow the visualization of a large number of nuclei.
Photomicrographs of a horizontal section showing fos-like positive nuclei in: (A) nucleus accumbens shell (Acb Sh) and bed nucleus of stria terminalis lateral division (BSTL) and (B) interstitial nucleus of posterior limb of the anterior commissure (IPAC), central amygdaloid nucleus (Ce) after 20 mg/kg IP of clozapine. Ac = anterior commissure, CPu = striatum.
FLI was already maximal after 10 mg/kg of clozapine in all areas examined (Table 1). Acute administration of clozapine did not induce FLI in areas belonging to the medial extended amygdala (medial amygdaloid nucleus and BST medial division) (data not shown).
Fos-Like-Immunoreactivity After Haloperidol
Acute administration of haloperidol (0.1 and 1 mg/kg SC) induced an increase in FLI in the Acb shell, dorsolateral striatum and IPAC, whereas a lower increase in FLI was observed in the Acb core (Table 1, Figures 3 and 5 ). In the prefrontal cortex, BST lateral division and SLEA, haloperidol did not increase FLI at any dosage (Table 1, Figure 3). In the central amygdaloid nucleus, a small increase in FLI was observed after 0.1 mg/kg, whereas, 1 mg/kg did not modify FLI (Table 1, Figure 3). Both in the central amygdaloid nucleus and BST lateral division, FLI was less pronounced after 1 mg/kg than 0.1 mg/kg (Table 1).
Number of fos-like positive nuclei in: bed nucleus of stria terminalis lateral division (BSTL), interstitial nucleus of the posterior limb of the anterior commissure (IPAC), sublenticular extended amygdala (SLEA), central amygdaloid nucleus (Amyg Ce) after vehicle or haloperidol. * p < .05, ** p < .001 vs. vehicle.
Similarly to clozapine, acute administration of haloperidol did not induce FLI in areas belonging to the medial extended amygdala (data not shown).
Control rats were treated with slightly acidified (pH 6.0) saline or with the control saline from SERENASE and the results were pooled together, because no different effects were observed in the two groups.
DISCUSSION
Acute administration of clozapine induced FLI in areas belonging to the central extended amygdala (central amygdaloid nucleus, IPAC, SLEA and BST lateral division). In contrast, haloperidol induced FLI in the IPAC but failed to increase FLI in SLEA and BST lateral division. Moreover, haloperidol induced a low increase of FLI in the central amygdaloid nucleus at lower but not higher doses.
To compare the results obtained with clozapine and haloperidol, the doses of drugs used were selected on the basis of previous studies and considering that clinically equivalent doses of the two drugs have approximately a 70-fold difference (Brunello et al. 1995).
From the early work of de Olmos (1972), which characterized a sublenticular corridor of cells between the central amygdaloid nucleus and the BST, and on the basis of several studies that have shown similarities of connections and neurochemistry between the central amygdaloid nucleus and the BST, the term “extended amygdala” has been applied to the continuum of cells that extends from the central and medial amygdaloid nucleus, through the SLEA and IPAC, to the BST (Alheid and Heimer 1988; Alheid et al. 1995; de Olmos et al. 1985; Heimer et al. 1997b).
Two partitions of the extended amygdala are recognized: the central, composed by the central amygdaloid nucleus, IPAC and BST lateral division; and the medial, composed of the medial amygdaloid nucleus and the BST medial division (Heimer et al. 1997b). These two partitions differ in their efferent targets, because the central, but not the medial, extended amygdala is connected to the Acb shell (Heimer et al. 1997b). The BST lateral division and the central amygdaloid nucleus receive, in fact, projections from the Acb shell; conversely, these two areas reciprocate these projections (Heimer et al. 1997a; Heimer et al. 1997b; Zahm and Brog 1992).
The SLEA can also be subdivided in a central and medial division; however, the cells composing these two parts are not well defined, and they can be differentiated only by using specific immunohistochemical markers (Heimer et al. 1997b); therefore, the two SLEA divisions were not distinguished in this study.
Because clozapine, but not haloperidol, induced FLI in the central extended amygdala, the present study might suggest that atypical and classical antipsychotics exert some of their therapeutic effects through different mechanisms that might be related to FLI induction in all these areas.
The only area of the extended amygdala in which haloperidol strongly increased FLI was the IPAC. This result, however, is probably attributable to the large rise in FLI induced by haloperidol in the striatum, from which IPAC, otherwise called fundus striati (Paxinos and Watson 1986), is not distinguished. Therefore, this result might not indicate a specific action of haloperidol in the extended amygdala. The only effect of haloperidol that could be regarded as specifically attributable to an effect on the extended amygdala was the increase of FLI observed in the central amygdaloid nucleus after low, but not high, doses. The reason why haloperidol did not affect FLI after the higher dose is not apparent; however, this result suggests that the increase observed after haloperidol in the central amygdaloid nucleus is limited and of low intensity.
Previous studies (Wang and Rebec 1996) have shown that clozapine blocked the electrical and behavioral excitatory effects of intra-amygdala infusion of amphetamine, whereas, haloperidol failed to reverse the increase in neuronal activity and only partially blocked the behavioral activation. Moreover, FLI studies (Sebens et al. 1995) have shown that clozapine induced a higher increase of FLI in the central amygdaloid nucleus, as compared to haloperidol. Our results, showing that clozapine and haloperidol differentially affected FLI in all central extended amygdala portions, provide evidence for a different role of the two drugs, not only in the amygdala, but also in the whole central extended amygdala.
Stressor stimuli processed by limbic forebrain circuits, such as restraint, induce FLI in mesolimbic areas (Senba and Ueyama 1997). Therefore, in the present study, stress was minimized by habituating the rats to handling and by keeping them in the animal room and in a familiar cage during the experiments. This procedure resulted in low levels of FLI in vehicle-treated rats, and this might explain the differences observed in the absolute number of fos-like positive neurons in the central amygdaloid nucleus between our results and those of previous researchers (Sebens et al. 1995).
The central amygdaloid nucleus and BST lateral division receive projections from the prelimbic and infralimbic cortices, which compose the prefrontal cortex (Cassell and Wright 1986; de Olmos et al. 1985). In these areas, as already reported (Deutch and Duman 1996; Fibiger 1994; MacGibbon et al. 1994; Merchant et al. 1996; Nguyen et al. 1992; Robertson and Fibiger 1992; Robertson et al. 1994; Wan et al. 1995), clozapine, but not haloperidol, increased FLI. Therefore, induction of FLI in the central extended amygdala, such as in the prefrontal cortex, might be a way to differentiate atypical from typical antipsychotics.
Both the prefrontal cortex and the amygdala play important roles in the processing and expression of emotional responses (Aggleton and Mishkin 1985; Aggleton 1993; Alheid and Heimer 1996). Studies in primates correlated the loss of social and affective behaviors with the removal of the amygdala (Aggleton 1993), whereas, studies in humans have described an impairment of social behaviors and in processing information having emotional significance after damage of the amygdala or the prefrontal cortex (Adolphs et al. 1995; Damasio et al. 1990; Damasio et al. 1994; Scott et al. 1997). The central amygdaloid nucleus is the output nucleus of the amygdala (Pitkänen et al. 1997) but also elaborates components of the integrated emotional behavior distinct from those elaborated in the basolateral nuclei (Gallager and Holland 1994; Killcross et al. 1997). Therefore, a prominent activation of FLI by clozapine, but not haloperidol, in the central extended amygdala, as reported by this study, might help to understand why clozapine is effective on such symptoms as blunted affect, social withdrawal and, in general, on the so-called negative symptoms of schizophrenia.
The therapeutic effects of clozapine have been attributed to an action on 5-HT2 and dopamine D4 receptors, whereas, the effects of haloperidol seems to be mediated by dopamine D2 and D3 receptors (Brunello et al. 1995). The central amygdaloid nucleus and BST lateral division receive large serotoninergic and noradrenergic innervation (Ma et al. 1991; Phelix et al. 1992; Sadikot and Parent 1990); moreover, the BST lateral division and the central amygdaloid nucleus, both directly and through the Acb shell and the IPAC, which belong to the most densely dopamine-innervated areas of the forebrain, are strongly affected by dopaminergic inputs (Freedman and Cassell 1994). The different efficacy of clozapine and haloperidol in inducing FLI in the central extended amygdala might be related to the complex innervation of these areas, which might mediate the effect of clozapine, but not haloperidol; however, the specific role of the different receptor types in the increase of FLI cannot be determined from the results of the present study.
In conclusion, the elevation of FLI induced by clozapine in the central extended amygdala provides evidence of the postulated importance of the extended amygdala in neuropsychiatric disorders characterized by affective disturbances and might be utilized as a tool to differentiate typical and atypical antipsychotics.
References
Adolphs R, Tranel D, Damasio H, Damasio AR . (1995): Fear and the human amygdala. J Neurosci 15: 5879–5891
Aggleton JP, Mishkin M . (1985): The amygdala: Sensory gateway to the emotions. In Plutchik R, Kellerman H (eds), Emotion: Theory, Research, and Experience. Orlando, FL, Academic, pp 281–299
Aggleton JP . (1993): The contribution of the amygdala to normal and abnormal emotional states. Trends Neurosci 16: 328–333
Alheid GF, Heimer L . (1988): New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: The striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience 27: 1–39
Alheid GF, de Olmos JS, Beltramino CA . (1995): Amygdala and extended amygdala. In Paxinos (ed), The Rat Nervous System, 2nd ed. San Diego, Academic Press, pp 495–578
Alheid GF, Heimer L . (1996): Theories of basal forebrain organization and the “emotional motor system.”. Prog Brain Res 107: 461–484
Beck CHM . (1994): Acute treatment with antidepressant drugs selectively increases the expression of c-fos in the rat brain. J Psychiat Neurosci 20: 25–32
Brunello N, Masotto C, Steardo L, Markstein R, Racagni G . (1995): New insights into the biology of schizophrenia through the mechanism of action of clozapine. Neuropsychopharmacology 13: 177–212
Cassell D, Wright DJ . (1986): Topography of projections from the medial prefrontal cortex to the amygdala in the rat. Brain Res Bull 17: 321–333
Damasio AR, Tranel D, Damasio H . (1990): Individuals with sociophatic behavior caused by frontal damage fail to respond automatically to social stimuli. Behav Brain Res 41: 81–94
Damasio H, Grabowski T, Frank R, Galaburda AM, Damasio AR . (1994): The return of Phineas Gage: Clues about the brain from the skull of a famous patient. Science 264: 1102–1105
de Olmos JS . (1972): The amygdaloid projection field in the rat as studied with the cupric silver method. In Eleftheriou BE (ed), The Neurobiology of the Amygdala. New York, Plenum Press, pp 145–204
de Olmos JS, Alheid GF, Beltramino CA . (1985): Amygdala. In Paxinos G (ed), The Rat Nervous System. Sydney, Academic Press, pp 223–334
Deutch AY, Duman RS . (1996): The effects of antipsychotic drugs on fos protein expression in the prefrontal cortex: Cellular localization and pharmacological characterization. Neuroscience 70: 377–389
Duncan GE, Knapp DJ, Johnson KB, Breese GR . (1996): Functional classification of antidepressants based on antagonism of swim stress-induced fos-like immunoreactivity. J Pharmacol Exp Ther 277: 1076–1080
Fibiger HC . (1994): Neuroanatomical targets of neuroleptic drugs as revealed by fos immunochemistry. J Clin Psychiat 55: 33–36
Freedman LJ, Cassell MD . (1994): Distribution of dopaminergic fibers in the central division of the extended amygdala of the rat. Brain Res 633: 243–252
Gallagher M, Chiba AA . (1996): The amygdala and emotion. Curr Opin Neurobiol 6: 221–227.
Gallager M, Holland PC . (1994): The amygdala complex: Multiple roles in associative learning and attention. Proc Natl Acad Sci 91: 11771–11776
Heimer L, Alheid GF, de Olmos JS, Groenewegen HJ, Haber SN, Harlan RE, Zahm DS . (1997a): The accumbens: Beyond the core-shell dichotomy. J Neuropsych Clin Neurosci 9: 354–381
Heimer L, Harlan RE, Alheid GF, Garcia MM, de Olmos JS . (1997b): Substantia Innominata: A notion which impedes clinical-anatomical correlations in neuropsychiatric disorders. Neuroscience 76: 957–1006
Kane J, Honigfeld G, Singer J, Meltzer H . (1988): Clozapine for the treatment-resistant schizophrenic: A double-blind comparison with chlorpromazine. Arch Gen Psychiat 45: 789–796
Killcross S, Robbins TW, Everitt BJ . (1997): Different types of fear-conditioned behavior mediated by separate nuclei within the amygdala. Nature 388: 377–380
Le Doux JE . (1995): Emotions: Clues for the brain. Ann Rev Psychol 46: 209–235
Ma QP, Yin GF, Ai MK, Hans JS . (1991): Serotonergic projections from the nucleus raphe dorsalis to the amygdala in the rat. Neurosci Lett 134: 21–24
MacGibbon GA, Lawlor PA, Bravo R, Dragunow M . (1994): Clozapine and haloperidol produce a differential pattern of immediate early gene expression in rat caudate-putamen, nucleus accumbens, lateral septum, and islands of Calleja. Mol Brain Res 23: 21–32
Merchant KM, Figur LM, Evans DL . (1996): Induction of c-fos mRNA in rat medial prefrontal cortex by antipsychotic drugs: Role of dopamine D2 and D3 receptors. Cerebr Cortex 6: 561–570
Morelli M, Pinna A, Ruiu S, Del Zompo M . (1999): Induction of Fos-like-immunoreactivity in the central extended amygdala by antidepressant drugs. Synapse 31: 1–4
Nguyen TV, Kosofsky BE, Birnbaum R, Cohen BM, Hyman SE . (1992): Differential expression of c-fos and Zif268 in rat striatum after haloperidol, clozapine, and amphetamine. Proc Natl Acad Sci USA 89: 4270–4274
Paxinos G, Watson C . (1986): The rat brain. In Stereotaxic Coordinates, 2nd ed. London, Academic Press
Phelix CF, Liposits Z, Paull WK . (1992): Monoamine innervation of bed nucleus of stria terminalis: An electron microscopic investigation. Brain Res Bull 28: 946–965
Pitkänen A, Savander V, LeDoux JE . (1997): Organization of intra-amygdaloid circuitries in the rat: An emerging framework for understanding functions of the amygdala. Trends Neurosci 20: 517–523
Robertson GS, Fibiger HC . (1992): Neuroleptics increase c-fos expression in the forebrain: Contrasting effects of haloperidol and clozapine. Neuroscience 46: 315–328
Robertson GS, Matsumura H, Fibiger HC . (1994): Induction patterns of fos-like immunoreactivity in the forebrain as predictors of atypical antipsychotic activity. J Parmacol Exp Ther 271: 1058–1066
Sadikot AF, Parent A . (1990): The monoaminergic innervation of the amygdala in the squirrel monkey: An immunohistochemical study. Neuroscience 36: 431–447
Sagar SM, Sharp FR, Curran T . (1988): Expression of c-fos protein in brain: Metabolic mapping at the cellular level. Science 240: 1328–1331
Scott SK, Young AW, Calder AJ, Hellawell DJ, Aggleton JP, Johnson M . (1997): Impaired auditory recognition of fear and danger following bilateral amygdala lesions. Nature 385: 254–257
Sebens JB, Koch T, Horst GJT, Korf J . (1995): Differential fos-protein induction in rat forebrain regions after acute and long-term haloperidol and clozapine treatment. Eur J Pharmacol 273: 175–182
Senba E, Ueyama T . (1997): Stress-induced expression of immediate early genes in the brain and peripheral organs of the rat. Neurosci Res 29: 183–207
Wan W, Ennulat DJ, Cohen BM . (1995): Acute administration of typical and atypical antipsychotic drugs induces distinctive patterns of fos expression in the rat forebrain. Brain Res 688: 95–104
Wang Z, Rebec GV . (1996): Amygdaloid neurons respond to clozapine rather than haloperidol in behaving rats pretreated with intra-amygdaloid amphetamine. Brain Res 711: 64–72
Zahm DS, Brog JS . (1992): On the significance of subterritories in the “accumbens” part of the rat ventral striatum. Neuroscience 50: 751–767
Acknowledgements
The authors thank Prof. Maria Del Zompo and Prof. Gaetano Di Chiara for the helpful discussions and Mrs. Adelaide Marchioni for typing the manuscript. The work was supported by funds from the Regione Autonoma della Sardegna, Assessorato alla Publica Istruzione.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pinna, A., Morelli, M. Differential Induction of Fos-Like-Immunoreactivity in the Extended Amygdala after Haloperidol and Clozapine. Neuropsychopharmacol 21, 93–100 (1999). https://doi.org/10.1016/S0893-133X(98)00136-5
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1016/S0893-133X(98)00136-5
Keywords
This article is cited by
-
Social defeat stress-specific increase in c-Fos expression in the extended amygdala in mice: Involvement of dopamine D1 receptor in the medial prefrontal cortex
Scientific Reports (2019)
-
Cells in midline thalamus, central amygdala, and nucleus accumbens responding specifically to antipsychotic drugs
Psychopharmacology (2003)