Abstract
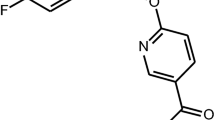
The benzodiazepines flunitrazepam, diazepam, and Ro 15-1788 and the β-carboline DMCM bind with equivalent affinity to the benzodiazepine binding site of GABAA receptors containing different α subunits (i.e., α1, α2, α3, or α5); whereas, the triazolopyridazine CL 218,872 and imidazopyridine zolpidem have higher affinity for α1 subunit-containing GABAA receptors. In the present study, the in vivo binding of [3H]Ro 15-1788 in mouse cerebellum and spinal cord was used to establish the occupancy of the benzodiazepine binding site of GABAA receptors containing primarily α1 and α2/α3 subunits, respectively. Thus, the nonselective compounds flunitrazepam, diazepam, and DMCM all produced a similar inhibition of binding in cerebellum and spinal cord (respective ID50 values of 0.2 to 0.3 mg/kg, 2 mg/kg, and 10 mg/kg IP); whereas, the α1 selective compounds CL 218,872 and zolpidem were more potent at inhibiting [3H]Ro 15-1788 binding in the cerebellum (ID50 values 4.5 mg/kg and 10 mg/kg IP) compared to the spinal cord (ID50 values 12 mg/kg and >30 mg/kg IP). Thus, the reduction of in vivo f [3H]Ro 15-1788 binding in tissues containing α1 and α2/α3 receptor populations reflects the in vitro affinities of subtype selective compounds and should help to interpret the behavioral profile of such compounds.
Similar content being viewed by others
Main
GABA is the major inhibitory neurotransmitter within the CNS (Rabow et al. 1995). Its actions are mediated via the ionotropic GABAA and GABAC receptors and the metabotropic GABAB receptor. To date, sixteen ionotropic GABA receptor subunit genes have been identified (α1–6, β1–3, γ1–3, δ, ρ1–2, and ε), with further diversity being introduced by the presence of alternatively spliced forms of certain of these subunits (Lüddens et al. 1995; Stephenson 1995; Davies et al. 1997; Whiting et al. 1997). Of these subunit genes, the ρ1–2 subunits constitute the GABAC receptor, which is found in the retina and arguably various regions of the brain (Johnston 1996); whereas, the GABAA receptor is generally considered to be a pentamer of α, β, and γ subunits, with the role of the δ and ε subunits being poorly understood (Lüddens et al. 1995; Stephenson 1995; McKernan and Whiting 1996; Davies et al. 1997; Whiting et al. 1997).
The GABAA receptor and its molecular structure and pharmacology has attracted considerable attention in view of it being the site of action of a number of compounds that can affect receptor function (i.e., chloride ion flux) either directly, by binding at the GABA site or within the ion channel, or indirectly, by binding to allosteric modulatory sites. Included in this latter class of compounds are the benzodiazepines, neurosteroids, barbiturates (such as pentobarbital), the anticonvulsant loreclezole, and some anesthetics (for example, etomidate and alphaxalone) (Sieghart 1995). Of these allosteric sites, the binding site for benzodiazepines has been most intensively studied, because of it's clinical relevance in mediating the anxiolytic, anticonvulsant, sedative-hypnotic, and muscle relaxant effects of such drugs as chlordiazepoxide, diazepam, and flunitrazepam.
A number of pharmacologically distinct benzodiazepine binding sites can be identified on the basis of their affinities for the “classical” benzodiazepines, such as diazepam and flunitrazepam, as well as the atypical benzodiazepines 2-oxoquazepam and Ro 15-4513 and nonbenzodiazepines, such as the triazolopyridazine CL 218,872, the imidazopyridine zolpidem, the β-carboline methyl β-carboline-3-carboxylate (β-CCM) and the cyclopyrrolones zopiclone and suriclone (Lüddens et al. 1995). The characterization of various cloned GABAA receptors expressed in either Xenopus oocytes or mammalian cells has defined the molecular basis of this pharmacological diversity (Rabow et al. 1995; Sieghart 1995). Hence, the benzodiazepine binding site requires the presence of an α and a γ subunit, with the pharmacology of the binding site of physiologically relevant GABAA receptors being determined largely by the type of α subunit present (Rabow et al. 1995; Sieghart 1995; McKernan and Whiting 1996).
More specifically, the α4 or α6 subunit renders GABAA receptors insensitive to “classical” benzodiazepines, such as diazepam. With respect to diazepam-sensitive GABAA receptors, the presence of an α1 subunit confers high affinity for CL 218,872 and zolpidem; whereas, α5 subunit-containing GABAA receptors have essentially no affinity for zolpidem. Receptors containing either an α2 or α3 subunit possess an intermediate affinity for zolpidem (Lüddens et al. 1995; Rabow et al. 1995; Sieghart 1995; McKernan and Whiting 1996). This pharmacological diversity is further exacerbated by the neuroanatomical heterogeneity of the distribution of these different GABAA receptor subtypes. Thus, although the cortex and hippocampus contain α1, α2, α3, α4, and α5 subunits, more restricted localizations are found in the cerebellum (primarily α1 and α6), spinal cord (primarily α2 and α3), and thalamus (primarily α1 and α4) (Wisden et al. 1991, 1992; Fritschy and Mohler 1995). Consistent with this anatomical distribution, in vitro pharmacological analysis of cerebellum and spinal cord GABAA receptors (Villiger 1984; Watanabe et al. 1985; Langer and Arbilla 1988; Benavides et al. 1988, 1992) suggests that the benzodiazepine pharmacology of the cerebellum and spinal cord are dictated largely by the α1 and α2 and α3 subunits, respectively.
The benzodiazepine binding site of α1, α2, α3, and α5 subunit-containing GABAA receptors can be labeled in vivo using radiolabeled Ro 15-1788 (Goeders and Kuhar 1985; Potier et al. 1988a, 1988b; Persson et al. 1985). However, within the cerebellum, binding is predominantly to α1 subunit-containing GABAA receptors because although the cerebellum is the major site of α6 subunit expression in the brain, Ro 15-1788 has a low affinity for this subpopulation of receptors. In addition, although [3H]Ro 15-1788 binds to the benzodiazepine site of GABAA receptors containing an α5 subunit, this population of receptors is expressed at relatively low levels throughout the brain as a whole, and in the cerebellum and spinal cord in particular (Fritschy and Mohler 1995; McKernan and Whiting 1996). Consequently, the binding of [3H]Ro 15-1788 to cerebellum and spinal cord in vivo represents binding to predominantly α1 and α2/α3 subunit-containing GABAA receptors, respectively.
The purpose of the present study was to establish whether the in vitro pharmacology of compounds relatively selective for GABAA receptors containing an α1 subunit, such as CL 218,872 and zolpidem, is reflected in vivo by differential occupancy in areas of the mouse CNS enriched in α1 (cerebellum) versus α2 and α3 (spinal cord) subunit-containing receptors.
METHODS
Materials
[3H]Ro 15-1788 (70-87 Ci/mmol) was obtained from NEN Life Science Products (Stevenage, UK). CL 218,872 was a gift from Lederle, and diazepam, flunitrazepam, methyl 6,7-dimethoxy-4-ethyl-β-carboline-3-carboxylate (DMCM) and zolpidem were purchased from Research Biochemicals International (St. Albans, UK).
In Vitro Radioligand Binding
The affinities of compounds binding at the benzodiazepine binding site of different α subunit-containing human recombinant GABAA receptors were measured essentially as described elsewhere (Hadingham et al. 1993). In brief, cells stably transfected with cDNA for the β3 and γ2 and various α subunits were harvested, and cell membranes prepared. Membranes (50 to 100 μg protein) were incubated in the presence of varying concentrations of compound for 1 h at 4°C in assay buffer (10 mM potassium phosphate buffer containing 100 mM KCl, pH 7.4) containing [3H]Ro 15-1788 (concentration in assay = 1.8 nM for α1 and α2 and/or 1.0 nM α3 and α5 subunit-containing receptors) in a final assay volume of 0.5 ml. Non-specific binding was defined using 10 μM flunitrazepam. The Ki values were calculated from the IC50 value according to the Cheng-Prussof equation (Cheng and Prussof 1973).
In Vivo Binding of [3H]Ro 15-1788
The methods used were essentially those of Goeders and Kuhar (1985) as modified to include the separation of membrane-bound from unbound radioactivity (Potier et al. 1988a, 1988b), although this separation of bound from free radioligand is not absolutely necessary (Facklam et al. 1992).
Time Course of Uptake of [3H]Ro 15-1788
[3H]Ro 15-1788 (70 to 87 Ci/mmol) was administered to male Swiss–Webster mice (26 to 30 g) by tail vein injection (5 μl/g of a 10 μCi/ml saline stock solution, equivalent to 1.5 μCi [3H]Ro 15-1788/30 g mouse). Mice were then killed at varying times (0.5 to 30 min) later, and the whole brain was removed, weighed, and rapidly homogenized in 10 volumes of ice-cold buffer (10 mM potassium phosphate buffer containing 100 mM KCl, pH 7.4). Aliquots of homogenate (200 μl) were either added directly to scintillation vials (total radioactivity; i.e., unbound and membrane-bound) or were filtered over Whatman GF/B filters (membrane-bound radioactivity). These were rapidly washed with 10 ml of ice-cold buffer, following which the filters were placed in vials, scintillation fluid was added, and radioactivity was measured using a scintillation counter.
Inhibition of In Vivo [3H]Ro 15-1788 Binding
Compounds were made up as either a suspension in 0.5% carboxymethylcellulose (CMC) or in solution in 70% polyethyleneglycol 300 and were administered IP 30 min prior to killing; whereas, [3H]Ro 15-1788 was given IV 3 min prior to killing. The cerebellum was then rapidly dissected from the brain, and the spinal cord was blown out of the spinal column using compressed air. Both tissues were weighed, rapidly homogenized, filtered, and counted as above.
Non-specific in vivo binding of [3H]Ro 15-1788 was estimated by measuring the membrane-bound radioactivity in mice pretreated for 30 min with 30 mg/kg flunitrazepam. In these animals, the radioactivity in 200 μl aliquots of homogenates of cerebellum and spinal cord (90 and 60 dpm, respectively) represented about 5% of the corresponding radioactivity seen in vehicle-treated animals (2,800 and 1,200 dpm, respectively).
RESULTS
Benziodiazepine-Site Ligand Affinities
In vitro, diazepam, flunitrazepam, and DMCM show no appreciable selectivity for the benzodiazepine binding site of different α subunit-containing GABAA receptors, with respective affinities across different recombinant GABAA receptors in the region of 10 to 20 nM, 2 to 5 nM, and 5 to 10 nM (Figure 1). In contrast, CL 218,872 and zolpidem had affinities for the α1 subunit-containing GABAA receptor of 57 and 27 nM, which represented a 6 to 34-fold and 14 to 20-fold selectivity for α1 versus α2– and α3–subunit-containing GABAA receptors for CL 218,872 and zolpidem, respectively.
Time Course of In Vivo [3H]Ro 15-1788 Binding
(Figure 2 ) shows the distribution of total (homogenate) and membrane-bound (filtered and washed homogenate) [3H]Ro 15-1788 at different times after IV injection. The time course of total and membrane-bound [3H]Ro 15-1788 were very similar, with radioactivity rapidly entering the brain (within 3 min) and then being cleared from the brain with a to.5 of around 8 min. Because maximal binding to whole brain membranes was observed after 3 min in all subsequent experiments, [3H]Ro 15-1788 was administered 3 min prior to killing.
Time course for the distribution of [3H]Ro 15-1788 in homogenates of whole brain of mice killed at various times after IV injection of radioligand. Total counts (▵) represent radioactivity in aliquots of unfiltered homogenate; whereas, membrane-bound counts (▴) were obtained following filtration of aliquots of homogenate over Whatman GF/B glass fiber filters. Values shown represent means ± SEM (n = 6/group). Based on these data, an interval of 3 min after radioligand injection was chosen as the time after which maximal membrane-bound [3H]Ro 15-1788 was achieved.
Reduction of In Vivo [3H]Ro 15-1788 Binding by Nonselective Ligands
(Figure 3 ) shows the reduction of in vivo [3H]Ro 15-1788 binding from the cerebellum and spinal cord produced by the nonselective compounds diazepam, flunitrazepam, and DMCM. All these compounds showed a comparable reduction in in vivo [3H]Ro 15-1788 binding (i.e., occupancy) in areas enriched in α1–subunit- (cerebellum) and α2/α3–subunit-containing (spinal cord) GABAA receptors.
The binding of [3H]Ro 15-1788 to mouse cerebellum (•) and spinal cord (○) membranes after IP injections of vehicle or various doses of the nonselective compounds diazepam, flunitrazepam, or DMCM. Values are expressed as a percentage of binding relative to vehicle-treated animals (vehicle = carboxymethylcellulose for diazepam and flunitrazepam or 70% polyethyleneglycol for DMCM) and are shown as means ± SEM (n = 4-8/group).
The dose required to produce a 50% reduction in in vivo binding of cerebellar and spinal cord [3H]Ro 15-1788 was around 0.2 to 0.3 mg/kg for flunitrazepam, 2 mg/kg for diazepam and 10 mg/kg for DMCM. However, the potency of these compounds was not related solely to their affinity. For example, diazepam has lower affinity for the different GABAA receptor subtypes than DMCM yet is fivefold more potent in vivo.
Reduction of In Vivo [3H]Ro 15-1788 Binding by α1-Selective Ligands
In contrast to nonselective compounds, the α1-selective compounds CL 218,872 and zolpidem produced a greater reduction of in vivo [3H]Ro 15-1788 binding (i.e., occupancy) in the α1 subunit-enriched cerebellum as compared to the predominantly α2/α3 subunit-containing spinal cord (Figure 4 ). Thus, the ID50 values for the inhibition of cerebellum and spinal cord [3H]Ro 15-1788 binding were 4.5 and 12 mg/kg for CL 218,872 and 10 and >30 mg/kg for zolpidem, respectively. Again, there was no relationship between in vitro affinity and in vivo potency, with CL 218,872 being around twice as potent as zolpidem at displacing in vivo [3H]Ro 15-1788 binding from cerebellum (α1 receptors), despite having twofold lower affinity for α1-containing receptors in vitro (affinities for CL 218,872 and zolpidem = 57 and 27 nM, respectively).
The binding of [3H]Ro 15-1788 to mouse cerebellum (•) and spinal cord (○) membranes after IP injections of vehicle or various doses of the α1-selective compounds CL 218,872 and zolpidem. Values are expressed as a percentage of binding relative to vehicle-treated animals (vehicle = 70% polyethyleneglycol for CL 218,872 or carboxymethylcellulose for zolpidem) and are shown as means ± SEM (n = 4-8/group).
The relatively flat displacement curve for zolpidem in the spinal cord (Figure 3) is comparable to that seen in the mouse hippocampus (Benavides et al. 1988) and rat spinal cord (Benavides et al. 1992), and presumably, it reflects the heterogeneity of GABAA receptors in these regions in contrast to the relatively homogeneous population of α1 subunit-containing benzodiazepine binding sites associated with cerebellar GABAA receptors.
DISCUSSION
The peak of both total and membrane-bound radioactivity was found to be 3 minutes after IV administration, in agreement with previous studies showing that [3H]benzodiazepines rapidly enter the brain (Goeders and Kuhar 1985; Ciliax et al. 1986; Potier et al. 1988b). The fact that more than 75% of the radioactivity in the brain is membrane bound suggests that the majority of [3H]Ro 15-1788 that gets into the brain is available for binding to the receptor and is not sequestered in pools unavailable for receptor binding. This is consistent with the low levels of nonspecific binding (<5%) as defined by doses of flunitrazepam that saturate the binding site and is in agreement with previous work (Potier et al. 1988a; Facklam et al. 1992). Taken together, these data demonstrate the utility of [3H]Ro 15-1788 as a radioligand with which to study benzodiazepine binding sites in vivo (Goeders and Kuhar 1985; Potier et al. 1988b).
The nonselective compounds diazepam, flunitrazepam, and DMCM, all of which have comparable affinities at different α subunit-containing GABAA receptors, had essentially the same ID50 for the displacement of [3H]Ro 15-1788 in cerebellum and spinal cord (Figure 3). A similar lack of regional selectivity for diazepam and flunitrazepam has been reported previously in various regions of the mouse (Potier et al. 1988b) and rat (Benavides et al. 1992) CNS. Moreover, the ID50 for flunitrazepam of 0.2 to 0.3 mg/kg is in good agreement with previous published values of 0.17 mg/kg (Benavides et al. 1988) and 0.5 mg/kg (Potier et al. 1988b). However, the ID50 for diazepam observed in the present study (2 mg/kg) is appreciably lower than previous estimates (10 mg/kg, Benavides et al. 1988).
Concerning α1 selective compounds, the in vitro selectivity of CL 218,872 and zolpidem for α1 versus α2 and α3 subunit-containing recombinant GABAA receptors (Figure 1) was reflected by a preferential in vivo occupancy of cerebellum versus spinal cord (α1 and α2/α3 subunit-containing tissues, respectively) benzodiazepine binding sites. Thus, lower doses of these α1-selective compounds were required to inhibit 50% of the in vivo binding of [3H]Ro 15-1788 in cerebellum compared to spinal cord. A comparable selectivity of in vivo binding has also been observed in mouse cerebellum versus cortex or hippocampus (Potier et al. 1988b) and in rat cerebellum versus spinal cord (Benavides et al. 1992). In addition, autoradiographic studies of in vivo [3H]Ro 15-1788 binding showed that the ID50 of zolpidem in a number of α1 subunit-containing brain regions was appreciably lower than in regions with a more heterogeneous distribution of GABAA receptor subtypes (Benavides et al. 1988).
Given the differences in drug formulation and dosing route between studies, the ID50 values for the displacement of in vivo [3H]Ro 15-1788 binding in the cerebellum and spinal cord by CL 218,872 (4.5 and 12 mg/kg, respectively) and zolpidem (10 and >30 mg/kg, respectively) agree reasonably well with values reported previously for CL 218,872 in the mouse cerebellum (12 mg/kg; Potier et al. 1988b) and for zolpidem in autoradiographic analysis of mouse cerebellum (3.6 mg/kg; Benavides et al. 1988). This degree of selectivity is also observed in the rat where respective ID50 values for cerebellum and spinal cord were 6 and 19 mg/kg for CL 218,872 and 7 and >100 mg/kg for zolpidem (Benavides et al. 1992), although direct comparisons between effective doses in mice and rats is complicated by the possible species differences in pharmacokinetics (e.g., bretazenil, Martin et al. 1988).
It should be noted that the differences in the cerebellum and spinal cord ID50 values for the reduction in in vivo [3H]Ro 15-1788 binding by CL 218,872 and zolpidem are generally less than the α1 selectivity of these compounds in vitro. For example, CL 218,872 had ID50 values of 4.5 and 12 mg/kg—an in vivo selectivity of less than threefold—whereas, the affinity of this compound at α1 subunit containing receptors in vitro is 20- and 35-fold higher than for α2 and α3 subunit-containing receptors, respectively. It is possible that this difference between in vitro affinity and in vivo potency could be attributable to different drug levels in the cerebellum and spinal cord. However, levels of zolpidem did not differ in rat cerebellum and spinal cord (Benavides et al. 1992), although this does not preclude the possibility of regional variations in CL 218,872 levels. The reason for this discrepancy between in vitro affinity and in vivo potency at α1 versus α2/α3 receptors in unclear but underscores the need to establish in vivo occupancy of subtype selective compounds to rationalize the behavioral properties of such drugs.
Compounds acting at the benzodiazepine site of the GABAA receptor produce a range of behavioral effects. The identification of subtypes of the GABAA receptor with distinct pharmacological and structural properties along with discrete neuroanatomical localizations has lead to the hypothesis that compounds that selectively interact with only some of these subtypes might possess only part of the range of effects associated with the nonselective benzodiazepines (Doble and Martin 1992). In addition to the in vivo occupancy, a further factor to be considered in understanding the mechanisms underlying the behavioral properties of a compound is the intrinsic efficacy of that compound (Facklam et al. 1992). Thus, for example, although compounds such as CL 218,872 and zolpidem selectively interact with the benzodiazepine site of α1 subunit-containing GABAA receptors, their different efficacies (partial and full agonists, respectively) results in distinct behavioral profiles (Lüddens et al. 1995). In this respect, the inhibition of in vivo binding of [3H]benzodiazepines has proved to be crucial in unraveling the relationships between the degree of receptor occupancy required by compounds of varying intrinsic efficacies to produce distinct behavioral effects (Duka et al. 1979; Benavides et al. 1988, 1992; Facklam et al. 1992; Giusti et al. 1993; Martin et al. 1993; Jones et al. 1994).
The problems of interpreting behavioral data in terms of receptor occupancy and intrinsic efficacy is given an added dimension when considering attributing distinct behavioral properties to compounds that selectively interact with particular GABAA receptor subtypes. In this regard, the fact that the occupancy of benzodiazepine binding sites in regions expressing different GABAA receptor populations reflects their in vitro binding affinity suggests that the displacement of in vivo [3H]Ro 15-1788 binding in cerebellum and spinal cord should prove to be a valuable tool in unraveling the complexities of the behavioral profiles of α1 or α2/α3 subtype-selective compounds.
References
Benavides J, Peny B, Dubois A, Perrault G, Morel E, Zivkovic B, Scatton B . (1988): In vivo interaction of zolpidem with central benzodiazepine (BZD) binding sites (as labeled by [3H]Ro 15-1788) in the mouse brain. Preferential affinity of zolpidem for the ω1 (BZD1) subtype. J Pharm Expt Ther 245: 1033–1041
Benavides J, Peny B, Durand A, Arbilla S, Scatton B . (1992): Comparative in vivo and in vitro regional selectivity of central ω (benzodiazepine) site ligands in inhibiting [3H]flumazenil binding in the rat central nervous system. J Pharmacol Expt Ther 263: 884–896
Cheng YC, Prussof WH . (1973): Relationship between the inhibition constant (Ki) and the concentration of inhibitor that causes 50% inhibition (IC50) of an enzymatic reaction. Biochem Pharmacol 22: 3099–3108
Ciliax BJ, Penney Jr JB, Young AB . (1986): In vivo [3H[flunitrazepam binding: Imaging of receptor regulation. J Pharmacol Expt Ther 238: 749–757
Davies PA, Hanna MC, Hales TG, Kirkness EF . (1997): Insensitivity to anaesthetic agents conferred by a class of GABAA receptor subunit. Nature 385: 820–823
Doble A, Martin IL . (1992): Multiple benzodiazepine receptors: No reason for anxiety. Trends Pharmacol Sci 13: 76–81
Duka T, Höllt V, Herz A . (1979): In vivo receptor occupancy by benzodiazepines and correlation with the pharmacological effect. Brain Res 179: 147–156
Facklam M, Schoch P, Haefely WE . (1992): Relationship between benzodiazepine receptor occupancy and functional effects in vivo of four ligands of differing intrinsic efficacies. J Pharmacol Expt Ther 261: 1113–1121
Fritschy J-M, Mohler H . (1995): GABAA-receptor heterogeneity in the adult rat brain: Differential regional and cellular distribution of seven major subunits. J Comp Neurol 359: 154–194
Giusti P, Ducic I, Puia G, Arban R, Walser A, Guidotti A, Costa E . (1993): Imidazenil: A new partial positive allosteric modulator of γ-aminobutyric acid (GABA) action at GABAA receptors. J Pharmacol Expt Ther 266: 1018–1028
Goeders NE, Kuhar MJ . (1985): Benzodiazepine receptor binding in vivo with [3H]-Ro 15-1788. Life Sci 37: 345–355
Hadingham KL, Wingrove P, Le Bourdelles B, Palmer KJ, Ragan CI, Whiting PJ . (1993): Cloning of cDNA sequences encoding human α2 and α3 γ-aminobutyric acidA receptor subunits and characterization of the benzodiazepine pharmacology of recombinant α1-, α2-, α3-, and α5-containing human γ-aminobutyric acidA receptors. Mol Pharmacol 43: 970–975
Johnston GAR . (1996): GABAc receptors: Relatively simple transmitter-gated ion channels? Trends Pharmacol Sci 17: 319–323
Jones GH, Schneider C, Schneider HH, Seidler J, Cole BJ, Stephens DN . (1994): Comparison of several benzodiazepine receptor ligands in two models of anxiolytic activity in the mouse: An analysis based on fractional receptor occupancies. Psychopharmacology 114: 191–199
Langer SZ, Arbilla S . (1988): Imidazopyridines as a tool for the characterization of benzodiazepine receptors: A proposal for a pharmacological classification as omega receptor subtypes. Pharmacol Biochem Behav 29: 763–766
Lüddens H, Korpi ER, Seeburg PH . (1995): GABAA/benzodiazepine receptor heterogeneity: Neurophysiological implications. Neuropharmacology 34: 245–254
Martin JR, Pieri L, Bonetti EP, Schaffner R, Burkard WP, Cumin R, Haefely WE . (1988): Ro 16-6028: A novel anxiolytic acting as a partial agonist at the benzodiazepine receptor. Pharmacopsychiatry 21: 360–362
Martin JR, Schoch P, Jenck F, Moreau J-L, Haefely WE . (1993): Pharmacological characterization of benzodiazepine receptor ligands with intrinsic efficacies ranging from high to zero. Psychopharmacology 111: 415–422
McKernan RM, Whiting PJ . (1996): Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci 19: 139–143
Persson A, Ehrin E, Eriksson L, Fade L, Hedstrom C-G, Litton J-E, Mindus P, Sedvall G . (1985): Imaging of 11C-labeled Ro 15-1788 binding to benzodiazepine receptors in human brain by positron emission tomography. J Psychiatr Res 4: 609–622
Potier M-C, Prado de Carvalho L, Dodd RH, Besselievre R, Rossier J . (1988a): In vivo binding of β-carbolines in mice: Regional differences and correlation of occupancy to pharmacological effects. Mol Pharmacol 34: 124–128
Potier M-C, Prado de Carvalho L, Dodd RH, Brown CL, Rossier J . (1988b): In vivo binding of (3H)Ro 15-1788 in mice: Comparison with the in vivo binding of (3H)flunitrazepam. Life Sci 43: 1287–1296
Rabow LE, Russek SJ, Farb DH . (1995): From ion currents to genomic analysis: Recent advances in GABAA receptor research. Synapse 21: 189–274
Sieghart W . (1995): Structure and pharmacology of γ-aminobutyric acidA receptor subtypes. Pharmacol Rev 47: 181–234
Stephenson FA . (1995): The GABAA receptors. Biochem J 310: 1–9
Villiger JW . (1984): CL 218,872 binding to benzodiazepine receptors in rat spinal cord: Modulation by γ-aminobutyric acid and evidence for receptor heterogeneity. J Neurochem 43: 903–905
Watanabe Y, Khatami S, Shibuya T, Salafsky B . (1985): Ontogenic properties of benzodiazepine receptor subtypes in the rat spinal cord. Eur J Pharmacol 109: 307–309
Whiting PJ, McAllister G, Vasilatis D, Bonnert TP, Heavens RP, Smith DW, Hewson L, O'Donnell R, Rigby MR, Sirinathsinghji DJS, Marshall G, Thompson SA, Wafford KA . (1997): Neuronally restricted RNA splicing regulates the expression of a novel GABAA receptor subunit conferring atypical functional properties. J Neurosci 17: 5027–5037
Wisden W, Gundlach AL, Barnard EA, Seeburg PH, Hunt SP . (1991): Distribution of GABAA receptor subunit mRNAs in rat lumbar spinal cord. Mol Brain Res 10: 179–183
Wisden W, Laurie DJ, Monyer H, Seeburg PH . (1992): The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci 12: 1040–1062
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Atack, J., Smith, A., Emms, F. et al. Regional Differences in the Inhibition of Mouse In Vivo [3H]Ro 15-1788 Binding Reflect Selectivity for α1 versus α2 and α3 Subunit-Containing GABAA Receptors. Neuropsychopharmacol 20, 255–262 (1999). https://doi.org/10.1016/S0893-133X(98)00052-9
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1016/S0893-133X(98)00052-9
Keywords
This article is cited by
-
Marine polyphenol phlorotannins promote non-rapid eye movement sleep in mice via the benzodiazepine site of the GABAA receptor
Psychopharmacology (2014)
-
GABA-A receptor impairment in cerebellar ataxia with anti-glutamic acid decarboxylase antibodies
Journal of Neurology (2013)
-
Long-lasting Modulation of Glutamatergic Transmission in VTA Dopamine Neurons after a Single Dose of Benzodiazepine Agonists
Neuropsychopharmacology (2009)
-
Dissociating anxiolytic and sedative effects of GABAAergic drugs using temperature and locomotor responses to acute stress
Psychopharmacology (2009)
-
Selective labelling of diazepam‐insensitive GABAA receptors in vivo using [3H]Ro 15‐4513
British Journal of Pharmacology (2005)