Abstract
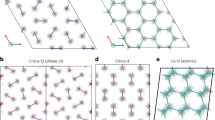
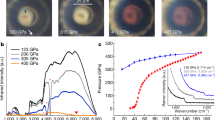
FROM a recent calculation1, it follows that the striking difference in properties between hydrogen and lithium is due to the great stability of the co-valent H–H link. Metallic hydrogen (the analogue of metallic lithium) would be formed from hydrogen atoms with an evolution of about 10 kcal. per gm. atom, but is only stable with respect to diatomic hydrogen molecules at pressures not less than 2.5 × 105 atmospheres.
Similar content being viewed by others
Article PDF
References
Wigner and Huntingdon, J. Chem. Phys., 3, 764 (1935).
cf. Ubbelohde, Trans. Far. Soc., 28, 275 (1932).
Ubbelohde, Trans. Far. Soc., 32, 525 (1936).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
UBBELOHDE, A. Expansion Pressures of Metallic Hydrogen and Deuterium. Nature 138, 845 (1936). https://doi.org/10.1038/138845a0
Issue Date:
DOI: https://doi.org/10.1038/138845a0
This article is cited by
-
Viscosity of Binary Mixtures
Nature (1937)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.