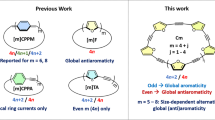
Abstract
THE nitroso-group in an aromatic nitroso compound appears to be chemically abnormal. Thus, in the case of nitrosobenzene, failure 1 to prepare salts or coordination compounds in which the nitroso-nitrogen acts as a donor atom, shows the accepted formula (1), with a tercovalent nitrogen atom, to be incorrect. Equality of the value of the dipole moment for this substance with that for nitrobenzene would indicate (2) as a possible formula, in which case the nitroso-group should be meta-directing in aromatic substitution reactions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Unpublished experiments.
Bamberger, Büsdorf, and Sand, Ber., 31, 1513; 1898.
Ingold, J.C.S., 516; 1925: and Bamberger, Ber., 30, 512; 1897.
Ingold, loc. cit.
Ham, Dissert., Zurich, 29; 1904.
Le Fèvre, J.C.S., 810; 1931.
Hammick and Illingworth, J.C.S., 2363; 1930.
Wieland, "Die Hydrazine", Stuttgart, 71; 1913.
Pauling, J. Amer. Chem. Soc., 53, 3234; 1931.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
LE FÈVRE, R. Orienting Power of the Nitroso-Group and the Formula of Nitrosobenzene. Nature 130, 400–401 (1932). https://doi.org/10.1038/130400a0
Issue Date:
DOI: https://doi.org/10.1038/130400a0
This article is cited by
-
Constitutions of Dinitrogen Tetroxide and Trioxide
Nature (1947)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.