Abstract
The serotonin 5-HT2A receptor is suspected to be involved in a number of psychiatric disorders, including schizophrenia. In particular, atypical antipsychotics have antagonistic effects on the 5-HT2A receptors, supporting a specific role of the 5-HT2A receptor in the pathophysiology of this disease. The aim of this study is to investigate cortical and subcortical 5-HT2A binding in neuroleptic-naive schizophrenic patients. Fifteen neuroleptic-naive patients diagnosed with schizophrenia (age 27.5±4.5 years), 11 men and 4 women, and 15 healthy control subjects matched for age (28.5±5.7 years) and gender underwent a 40 min positron emission tomography (PET) study using the 5-HT2A antagonist, [18F]altanserin, as a radioligand. PET images were co-registered to 3 T magnetic resonance images (MRIs) for each individual subject, and ROIs were applied automatically onto the individual MRIs and PET images. The cerebellum was used as a reference region. The binding potential of specific tracer binding (BPp) was used as the outcome measure. No significant difference was seen in cortical receptor distribution between patients and controls. An increase in 5-HT2A receptor binding in the caudate nucleus was detected in the group of schizophrenic patients (0.7±0.1) when compared to the healthy controls (0.5±0.3) (p=0.02). Our results confirm other in vivo findings of no difference in cortical 5-HT2A receptor binding between first-episode antipsychotic-naive schizophrenic patients and age- and gender-matched healthy control subjects. However, a preliminary finding of increased 5-HT2A binding in the caudate nucleus requires further investigation to explore the relation of subcortical and cortical 5-HT2A receptor binding.
Similar content being viewed by others
INTRODUCTION
A role for serotonin in the pathophysiology of schizophrenia is supported by different observations. Gaddum (1954) described that the hallucinogenic drug lysergic acid diethylamide had structural similarity to serotonin and could cause or exacerbate psychotic symptoms. These early findings led to the hypothesis that serotonin was implicated in the pathophysiology of schizophrenia. Further support for this comes from the notion that most atypical antipsychotic drugs (AAPDs) antagonize the serotonin 2A (5-HT2A) receptor. The affinity of AAPDs as determined in vitro (Meltzer et al, 1989) and in vivo (Zhang and Bymaster, 1999) is often higher for 5-HT2A than D2 receptors. Finally, post-mortem studies suggest a serotonergic dysfunction in cortical areas in schizophrenia. Eleven (Arora and Meltzer, 1991; Bennett, 1979; Burnet et al, 1996; Dean and Hayes, 1996; Dean et al, 1998, 1999; Gurevich and Joyce, 1997; Laruelle et al, 1993; Matsumoto et al, 2005; Mita et al, 1986; Pralong et al, 2000) out of 15 (Dean et al, 1996; Joyce et al, 1993; Reynolds et al, 1983; Whitaker et al, 1981) post-mortem studies of brains of schizophrenic patients have reported decreased 5-HT2A/C density in cortical areas, especially in the frontal cortex. Only two studies have addressed the subcortical 5-HT2A density in post-mortem material: Joyce et al (1993) found an increased 5-HT2A density in the ventral putamen and nucleus accumbens, whereas Matsumoto et al (2005) reported no significant difference in striatum between controls and patients suffering from schizophrenia.
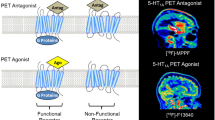
Investigations with in vivo imaging techniques have not supported post-mortem findings of a cortical decrease in 5-HT2A receptor binding in schizophrenia. Three positron emission tomography (PET) studies showed no difference in 5-HT2A/C receptor density between schizophrenic patients and controls (Lewis et al, 1999; Okubo et al, 2000; Trichard et al, 1998). Only one study revealed a decreased 5-HT2A binding potential in the frontal cortex of six neuroleptic-naive schizophrenic subjects when compared to healthy controls (Ngan et al, 2000). The latter publication was based on a voxel-based image analysis, whereas the three prior ones were based on a region-based analysis. All four previous studies were performed on a limited number of patients and only some of them were antipsychotic-naive. Three of the studies used [18F]setoperone as a 5-HT2A tracer, whereas one study used [11C]N-methylspiperone. Owing to a relatively poor selectivity of both radiotracers for the 5-HT2A receptor, these studies are limited to the detection of receptor binding only in cortical areas. In conjunction with the poor selectivity of the tracers, a lower ratio of 5-HT2A receptors to D2 receptors in subcortical areas compared to cortical areas makes these ligands inadequate to measure subcortical binding. Today, two other specific 5-HT2A radioligands are available: [11C]MDL 100,907 and [18F]altanserin. [18F]altanserin has a 200- to 500-fold 5-HT2A/D2 selectivity measured as 1/(5-HT2A Ki/D2 Ki)=1/(0.13–0.3/62 nM)=1/(0.002–0.005) (Kristiansen et al, 2005; Tan et al, 1999), making it between 8 and 50 times more selective for the 5-HT2A receptors than [18F]setoperone ((1/(1/10–25 nM)=1/(0.1–0.04)=10–25-fold 5-HT2A/D2 selectivity (Lewis et al, 1999)). In addition, the affinity of [18F]altanserin for the 5-HT2A receptor is at least 20-fold higher than for other 5-HT subtypes (Tan et al, 1999). We have previously demonstrated that [18F]altanserin PET with a bolus infusion design is a highly reproducible method for reliable quantification of 5-HT2A receptor binding (Haugbøl et al, 2007).
The aim of the present PET study was to investigate cortical and subcortical 5-HT2A receptor binding in a group of first-episode antipsychotic-naive schizophrenic patients and matched healthy controls using [18F]altanserin PET.
MATERIALS AND METHODS
Participants
Fifteen patients (11 men and 4 women) were recruited after voluntary first-time referral to a psychiatric unit of one of the affiliated university hospitals in the Copenhagen area (Bisbebjerg Hospital, Rigshospitalet, Psychiatric University Centre Glostrup or Psychiatric University Centre Gentofte). The study was approved by the Ethics Committee of Copenhagen and Frederiksberg ((KF)11-061/03). The subjects participated after receiving a full explanation of the study and providing written informed consent according to the declaration of Helsinki II.
The patients included fulfilled diagnostic criteria for schizophrenia according to both ICD-10 and DSM IV. All patients were antipsychotic-naive at the time of investigation. Diagnosis was verified by means of the structured clinical interview SCAN 2.1 (Schedules for Clinical Assessment in Neuropsychiatry). The severity of symptoms in subjects was assessed with Positive and Negative Syndrome Scale (PANSS). All interviews were recorded on video for validation purposes.
Fifteen healthy control subjects matched for age, gender, and ethnicity were recruited from the community by advertisement. None of the healthy control subjects had either a history of present or prior psychiatric disorder or had ever used any psychotropic medication as determined by SCAN interviews.
Four patients had prior (n=3) or present (n=1) use of antidepressant medication (in all cases selective serotonin reuptake inhibitors). Current use of benzodiazepines was allowed, albeit not on the day of the PET scans. Except for one, none of the patients used any drugs of abuse or fulfilled ICD-10 or DSM IV criteria for either drug abuse or drug dependence by the time of inclusion. None of the healthy controls or any of the patients had a history of significant head injury or non-psychiatric disorder. Both healthy controls and patients had a normal neurological interview and examination.
Magnetic Resonance Imaging
High-resolution 3D T1-weighted, sagittal, spoiled gradient echo scans (MPRAGE) of the head (TI/TE/TR=800/3.93/1540 ms, flip angle 9°; matrix: 256 × 256; 192 slices) using an eight-channel head array coil were acquired in all subjects on a 3 T TRIO scanner (Siemens, Erlangen, Germany) at the MR department of the Copenhagen University Hospital, Hvidovre, Denmark.
[18F]altanserin PET Studies
Radiosynthesis and administration
The radiosynthesis of [18F]altanserin was according to the method described previously by Lemaire et al (1991). Quality control was performed using thin-layer chromatography and high-performance liquid chromatography (HPLC). The absence of residual solvents (methanol, THF, and DMSO) in the final formulation was confirmed by 1H NMR. For each PET study, 0.3–3.5 GBq of [18F]altanserin was produced with a radiochemical yield greater than 95% and a mean specific activity of 52.4±34.0 GBq/μmol. Catheters were inserted in both cubital veins for tracer infusion and blood sampling, respectively. [18F]altanserin was administrated as a combination of a bolus injection followed by continuous infusion to obtain steady state of the tracer in blood and tissue. The bolus-infusion ratio was 1.75 h, as previously described (Pinborg et al, 2003). Subjects received the maximum dose of 3.7 MBq/kg body weight [18F]altanserin.
Imaging
PET scans were acquired in tracer steady-state conditions with an 18-ring GE-Advance scanner (GE, Milwaukee, WI, USA), operating in 3D-acquisition mode, producing 35 image slices with an interslice distance of 4.25 mm. The total axial field of view was 15.2 cm with an approximate in-plane resolution down to 5 mm. During steady state, the fraction of unmetabolized tracer in venous plasma was determined at five time points using HPLC analysis. Reconstruction, attenuation, and scatter correction procedures were conducted according to Pinborg et al (2003).
Ninety minutes after the bolus injection of [18F]altanserin, the subjects were placed in the scanner. Subjects were aligned in the scanner using a laser system so that the detectors were parallel to the orbitomeatal line and positioned to include the cerebellum in the field of view using a short 2 min transmission scan. An individual head holder was made to ensure relative immobility. All subjects were scanned in a resting state. A 10-min transmission scan was obtained for correction of tissue attenuation using retractable 68Ge/68Ga pin sources. The transmission scans were corrected for tracer activity by a 5-min emission scan performed in 2D mode. Dynamic 3D emission scans (five frames of 8 min) were started 120 min after tracer administration.
Data were reconstructed into a sequence of 128 × 128 × 35 voxel matrices, each voxel measuring 2.0 × 2.0 × 4.25 mm, with software provided by the manufacturer. A 3D reprojection algorithm with a transaxial Hann filter (6 mm) and an axial ramp filter (8.5 mm) was applied. Corrections for dead-time, attenuation, and scatter were performed.
Blood samples
Five venous blood samples were drawn at mid-scan times 4, 12, 20, 28, and 36 min after starting the dynamic scanning sequence. The samples were immediately centrifuged, and 0.5 ml of plasma was counted in a well counter for determination of radioactivity. Three of the five blood samples drawn at 4, 20, and 36 min were also analyzed for percentage of parent compound ([18F]altanserin) using reverse-phase HPLC following the procedure described by Pinborg et al (2003).
In addition, the free fraction of [18F]altanserin in plasma, f1, was estimated using equilibrium dialysis, following a modified procedure by Videbaek et al (1993). The dialysis was performed using Teflon-coated dialysis chambers (Harvard Bioscience, Amika, Holliston, MA, USA) with a cellulose membrane that retains proteins >10 000 Da. A small amount of [18F]altanserin (approximately 1 MBq) was added to 10 ml plasma samples drawn from the subjects. A 500 μl portion of plasma was then dialyzed at 37°C for 3 h against an equal volume of buffer, since pilot studies had shown that 3 h equilibration time yielded stable values. The buffer consisted of 135 mM NaCl, 3.0 mM KCl, 1.2 nM CaCl2, 1.0 mM MgCl2, and 2.0 mM phosphate (pH 7.4). After the dialysis, 400 μl of plasma and buffer were counted in a well counter, and f1 of [18F]altanserin was calculated as the ratio of DPMbuffer/DPMplasma.
Data Analysis
MR/PET co-registration
PET images and magnetic resonance images (MRIs) were co-registered using a Matlab (Mathworks Inc., Natick, MA, USA)-based program (Willendrup et al, 2004), where PET images and MRIs are brought to fit through manual translation and rotation of the PET image with subsequent visual inspection in three planes (Adams et al, 2004).
Volumes of interest and partial volume correction
Volumes of interest (VOIs) were automatically delineated on each individual's transaxial MRI slices in a strictly user-independent manner (Svarer et al, 2005). With this approach, a template set of 10 MRIs is automatically co-registered to a new subject's MRI. The identified transformation parameters are used to define VOIs in the new subject MRI space, and through the co-registering these VOIs are transferred onto the PET images. The investigated regions included frontal cortex (consisting of orbitofrontal, medial inferior frontal, and superior frontal subregions), anterior cingulate, posterior cingulate, insula, superior temporal cortex, medial inferior temporal cortex, sensory motor cortex, parietal cortex, occipital cortex, putamen/pallidus, thalamus, caudate nucleus, and cerebellum. For normalization purposes (see below), a global neocortical region was created for each subject. It consisted of a volume-weighted average of the binding potentials from the cortical regions listed above.
To enable partial volume correction of the PET data, MRIs, corrected for RF inhomogeneities using the N3 software (Sled et al, 1998), were segmented into gray matter, white matter, and cerebrospinal fluid tissue classes using SPM2 (Wellcome Department of Cognitive Neurology, London, UK). Partial volume correction was performed according to Quarantelli et al (2004). The white matter value was extracted as the mean voxel value from a predominantly white matter VOI (mid-brain) in the uncorrected PET image. As the MRIs and PET images have been co-registered, it is possible to calculate the number of gray matter voxels in native subject space for each VOI, and this is reported as the gray matter volume for the VOI.
Quantification of the 5-HT2A receptor binding
The outcome parameter was the binding potential of specific tracer binding (BPp). The cerebellum was used as a reference region, since it represents nonspecific binding only (Pinborg et al, 2003). In steady state, BPP is defined as

where CROI and CReference are steady-state mean count density in the VOI and in the reference region, respectively, CPlasma is the steady-state activity of non-metabolized tracer in plasma, f1 is the free fraction of radiotracer, Bmax is the density of receptor sites available for tracer binding, and Kd is the affinity constant of the radiotracer to the receptor.
Statistics
Between-group (patients, controls) comparisons of all reported outcome measures were performed using parametric analysis after verifying that the data were normally distributed according to the Kolmogorov–Smirnov test. P-values from unpaired two-tailed t-test were reported; p=0.05 was employed as the level of significance. Post hoc linear regression analysis was performed with caudate 5-HT2A receptor binding as the dependent variable and PANSS scores and age as independent variables. All analyses were performed using the statistical software SAS 9.1.
RESULTS
As shown in Table 1, no significant differences were observed in age, body mass index, injected dose, plasma free fraction, specific radioactivity of [18F]altanserin, and nonspecific binding between the two groups.
As illustrated in Table 2, two-tailed unpaired t-test revealed no between-group differences in 5-HT2A BPP either in any of the cortical regions or in the thalamus or putamen. However, schizophrenic patients displayed a significantly higher 5-HT2A BPP in the caudate nucleus than controls (0.72±0.15 vs 0.52±0.27, p=0.02, uncorrected). Exclusion of the subjects with prior antidepressant treatment (four patients) and illegal drug use (one patient) and their matched controls did not alter these results.
In the patient group, the positive, negative, and general symptom scores as assessed with PANSS were 18.6±5.0, 20.6±6.6, and 36.0±7.1, respectively. Post hoc linear regression analysis with adjustment for age did not reveal any significant relationship between caudate 5-HT2A receptor binding and positive, negative, or general PANSS scores. Further, there was no statistically significant group difference in the ratio between uncorrected and partial volume-corrected BPp values in the caudate (0.4±0.1 vs 0.5±0.2 in controls, p=0.57).
There were no between-group differences in total gray matter volume in the caudate (2.3±0.3 vs 2.3±0.2 ml in controls, p=0.49) or in any of the other regions of interest (data not shown).
DISCUSSION
The number of antipsychotic-naive first-episode schizophrenic patients in our study is so far the largest sample examined. Earlier studies have included 6 (Ngan et al, 2000), 7 (Trichard et al, 1998), 10 (Okubo et al, 2000), and 10 (Lewis et al, 1999) patients. Our data are in agreement with most of the previously published PET studies (Lewis et al, 1999; Okubo et al, 2000; Trichard et al, 1998) where no significant difference in cortical 5-HT2A receptor binding was found in schizophrenic patients as compared to healthy controls. The data of Lewis et al (1999) were later reanalyzed with a voxel-based approach (Verhoeff et al, 2000) that confirmed the outcome of the region-based analysis. In contrast, Ngan et al (2000) reported a decreased 5-HT2A binding in the frontal cortex of six neuroleptic-naive schizophrenic subjects, and such a difference was also observed in a recent PET study of six subjects with elevated risk of developing schizophrenia (Hurlemann et al, 2005).
Despite some inconsistency in post-mortem data, the majority of post-mortem studies suggest a decreased cortical 5-HT2A receptor binding in patients with schizophrenia. This is in contrast to the outcome of most other in vivo studies, including our own. This discrepancy might be caused by the influence of antipsychotic drug treatment and cause of death in the studies of post-mortem brain tissues. Suicide as a cause of death has been associated with increased post-mortem 5-HT2A receptor density, especially in younger cohorts (Oquendo et al, 2006). At the same time, treatment with antipsychotic drugs that antagonize the 5-HT2A receptor decreases levels of expression of the receptor (for a review, see Dean, 2003). These factors are therefore important to take into account when interpreting reports of 5-HT2A receptor levels in post-mortem tissue from schizophrenic patients. In vivo PET data from antipsychotic-naive patients are spared from such confounders and might be more reliable in assessing receptor regulations in schizophrenia. On the other hand, autoradiographic post-mortem studies, in contrast to PET imaging, do allow for detection of differences in receptor binding within cortical cell layers. In conclusion, at present no firm conclusions can be made on cortical 5-HT2A receptor binding in patients with schizophrenia.
Four of the patients in the present study had prior (n=3) or present (n=1) use of antidepressant medication and one patient had illegal drug use by the time of inclusion. However, a post hoc analysis, performed after removing these patients and their controls from the analysis, did not change the results.
In patients with schizophrenia, we found an increased 5-HT2A receptor binding in the caudate nucleus, whereas no differences were seen in the thalamus or putamen. Owing to lack of selectivity of the radioligands employed in earlier studies, this is the first study to assess in vivo subcortical 5-HT2A receptor binding in schizophrenic patients. The 5-HT2A receptor density in subcortical brain regions is only modest, and accordingly for those brain regions a larger sample is required to exclude type II errors (Haugbøl et al, 2007). Furthermore, no relationship between severity of psychotic symptoms assessed with PANSS and caudate 5-HT2A receptor binding could be established. For the above reasons and since no corrections for multiple comparisons were made, we consider our finding of an increased 5-HT2A receptor binding in the caudate nucleus in schizophrenic patients as preliminary.
To assess any eventual regional pattern differences in further detail, we also took an additional approach. We and others have observed that cerebral 5-HT2A receptor binding displays a high degree of autocorrelation, so that a large fraction of the interindividual variability can be explained by a factor difference. To assess the subcortical binding relative to the cortical binding, we normalized the subcortical regions with a volume-weighted average of cortical BPP and evaluated the within-group difference. Using these normalized values, the difference in caudate values turned out to be even more significant (p=0.001).
If confirmed, an increase in 5-HT2A receptor levels in schizophrenic patients could support a direct role of blockade of striatal 5-HT2A receptors in the mechanisms of action of a number of second-generation antipsychotics—in addition to the assumed indirect effects via modulation of cortical as well as striatal dopamine activity by 5-HT2A receptor blockade (Glenthoj et al, 1999; Meltzer et al, 2003; Svensson et al, 1995). An independent role of 5-HT2A receptor blockade in the mechanisms of action of second-generation antipsychotics is also supported by data demonstrating an association between polymorphisms in the promoter and coding regions of the 5-HT2A receptor gene and schizophrenia and/or the response to treatment with clozapine (Abdolmaleky et al, 2004; Arranz et al, 1998a, 1998b; Masellis et al, 1998). Furthermore, second-generation antipsychotics are also effective as an add-on to treatment with selective serotonin reuptake inhibitors in patients with obsessive–compulsive disorder (Denys et al, 2007; Skapinakis et al, 2007), and we have previously found increased 5-HT2A receptor binding in the caudate nuclei of patients with this disease (Adams et al, 2005). The finding of increased 5-HT2A receptor binding in the caudate nuclei in patients with obsessive–compulsive disorder as well as in patients with schizophrenia may suggest a common pathophysiological mechanism in agreement with the frequent occurrence of obsessive–compulsive symptoms in schizophrenic patients (Kayahan et al, 2005a, 2005b). If an increase in striatal 5-HT2A receptor binding is confirmed, the effects could still, however, be mediated through interactions with the dopaminergic system.
An increased 5-HT2A receptor binding in the caudate nucleus, as suggested by the preliminary data in the present study, might alternatively result from a compensatory upregulation of 5-HT2A receptors in response to altered serotonin levels. In addition, a defect in the medial raphe-cortico-striatal serotonergic circuit has been suggested to result in disinhibition of the mesolimbic DA system, a mechanism likewise suspected to play an important role in the pathophysiology of schizophrenia (Abi-Dargham et al, 1997). Because of a paradoxical regulation of the serotonin 5-HT2A receptor (Gray and Roth, 2001), antagonism would lead to downregulation of the receptor, thereby normalizing its levels. The changes in striatal 5-HT2A receptor density in schizophrenia are in accordance with the post-mortem findings by Joyce et al (1993). By contrast, Matsumoto et al (2005) did not find differences in striatal 5-HT2A receptor density between schizophrenic subjects and controls; likewise, no change in striatal 5-HT2A receptor binding was seen in a smaller study of six subjects at risk of schizophrenia (Hurlemann et al, 2005).
CONCLUSION
The present study is the first PET study exploring striatal as well as cortical 5-HT2A receptor binding in first-episode antipsychotic-naive schizophrenic patients. It is also the largest in vivo study on 5-HT2A receptors in those patients until now. We find no difference in the cortical regions or in the thalamus or putamen between the two groups, whereas an increased 5-HT2A receptor binding is detected in the caudate nucleus in 15 first-episode antipsychotic-naive schizophrenic patients when compared to age- and gender-matched healthy control subjects. This supports a direct or an indirect role of striatal 5-HT2A receptors in the pathophysiology of schizophrenia and in the mechanisms of action of many atypical antipsychotics. Further studies are, however, needed to explore the relation of subcortical and cortical 5-HT2A receptor activity to psychopathology, information processing, and other neurobiological measures.
References
Abdolmaleky HM, Faraone SV, Glatt SJ, Tsuang MT (2004). Meta-analysis of association between the T102C polymorphism of the 5HT2a receptor gene and schizophrenia. Schizophr Res 67: 53–62.
Abi-Dargham A, Laruelle M, Aghajanian GK, Charney D, Krystal J (1997). The role of serotonin in the pathophysiology and treatment of schizophrenia. J Neuropsychiatry Clin Neurosci 9: 1–17.
Adams KH, Pinborg LH, Svarer C, Hasselbalch SG, Holm S, Haugbøl S et al (2004). A database of [(18)F]-altanserin binding to 5-HT(2A) receptors in normal volunteers: normative data and relationship to physiological and demographic variables. NeuroImage 21: 1105–1113.
Adams KH, Hansen ES, Pinborg LH, Hasselbalch SG, Svarer C, Holm S et al (2005). Patients with obsessive–compulsive disorder have increased 5-HT2A receptor binding in the caudate nuclei. Int J Neuropsychopharmacol 3: 391–401.
Arora RC, Meltzer HY (1991). Serotonin2 (5-HT2) receptor binding in frontal cortex of schizophrenic patients. J Neural Transm 85: 19–29.
Arranz MJ, Munro J, Owen MJ, Spurlock G, Sham PC, Zhao J et al (1998a). Evidence for association between polymorphisms in the promoter and coding regions of the 5-HT2A receptor gene and response to clozapine. Mol Psychiatry 3: 61–66.
Arranz MJ, Munro J, Sham P, Kirov G, Murray RM, Collier DA et al (1998b). Meta-analysis of studies on genetic variation in 5-HT2A receptors and clozapine response. Schizophr Res 32: 93–99.
Bennett JP, Enna SJ, Bylund DB, Gillin JC, Wyatt RJ, Snyder SH (1979). Neurotransmitter receptors in frontal cortex of schizophrenics. Arch Gen Psychiatry 36: 927–934.
Burnet PW, Eastwood SL, Harrison PJ (1996). 5-HT1A and 5-HT2A receptor mRNAs and binding site densities are differentially altered in schizophrenia. Neuropsychopharmacology 15: 442–455.
Dean B (2003). The cortical serotonin2A receptor and the pathology of schizophrenia: a likely accomplice. J Neurochem 85: 1–13.
Dean B, Hayes W (1996). Decreased frontal cortical serotonin2A receptors in schizophrenia. Schizophr Res 21: 133–139.
Dean B, Hayes W, Hill C, Copolov D (1998). Decreased serotonin2A receptors in Brodmann's area 9 from schizophrenic subjects. A pathological or pharmacological phenomenon? Mol Chem Neuropathol 34: 133–145.
Dean B, Hayes W, Opeskin K, Naylor L, Pavey G, Hill C et al (1996). Serotonin2 receptors and the serotonin transporter in the schizophrenic brain. Behav Brain Res 73: 169–175.
Dean B, Hussain T, Hayes W, Scarr E, Kitsoulis S, Hill C et al (1999). Changes in serotonin2A and GABA(A) receptors in schizophrenia: studies on the human dorsolateral prefrontal cortex. J Neurochem 72: 1593–1599.
Denys D, Fineberg N, Carey PD, Stein DJ (2007). Quetiapine addition in obsessive–compulsive disorder: is treatment outcome affected by type and dose of serotonin reuptake inhibitors? Biol Psychiatry 61: 412–414.
Gaddum JH (1954). Drug antagonistic to 5-hydroxytryptamine. In: Wolstenholme GW (ed). Ciba Foundation Symposium on Hypertension. Little Brown and Company: Boston, pp 75–77.
Glenthoj BY, Mackeprang T, Bille AE, Hemmingsen RP (1999). [Transmitter dysfunction in patients with schizophrenia. Significance for cognitive functioning and treatment]. Ugeskr Laeger 161: 1391–1398.
Gray JA, Roth BL (2001). Paradoxical trafficking and regulation of 5-HT(2A) receptors by agonists and antagonists. Brain Res Bull 56: 441–451.
Gurevich EV, Joyce JN (1997). Alterations in the cortical serotonergic system in schizophrenia: a postmortem study. Biol Psychiatry 42: 529–545.
Haugbøl S, Pinborg LH, Arfan HM, Frøkjær VM, Madsen J, Dyrby TB et al (2007). Reproducibility of 5-HT(2A) receptor measurements and sample size estimations with [(18)F]altanserin PET using a bolus/infusion approach. Eur J Nucl Med Mol Imaging 34: 910–915.
Hurlemann R, Boy C, Meyer PT, Scherk H, Wagner M, Herzog H et al (2005). Decreased prefrontal 5-HT2A receptor binding in subjects at enhanced risk for schizophrenia. Anat Embryol (Berl) 210: 519–523.
Joyce JN, Shane A, Lexow N, Winokur A, Casanova MF, Kleinman JE (1993). Serotonin uptake sites and serotonin receptors are altered in the limbic system of schizophrenics. Neuropsychopharmacology 8: 315–336.
Kayahan B, Ozturk O, Veznedaroglu B (2005a). Obsessive–compulsive symptoms in schizophrenia. Turk Psikiyatri Derg 16: 205–215.
Kayahan B, Ozturk O, Veznedaroglu B, Eraslan D (2005b). Obsessive–compulsive symptoms in schizophrenia: prevalence and clinical correlates. Psychiatry Clin Neurosci 59: 291–295.
Kristiansen H, Elfving B, Plenge P, Pinborg LH, Gillings N, Knudsen GM (2005). Binding characteristics of the 5-HT2A receptor antagonists altanserin and MDL 100907. Synapse 58: 249–257.
Laruelle M, Abi-Dargham A, Casanova MF, Toti R, Weinberger DR, Kleinman JE (1993). Selective abnormalities of prefrontal serotonergic receptors in schizophrenia: a postmortem study. Arch Gen Psychiatry 50: 810–818.
Lemaire C, Cantineau R, Guillaume M, Plenevaux A, Christiaens L (1991). Fluorine-18-altanserin: a radioligand for the study of serotonin receptors with PET: radiolabeling and in vivo biologic behavior in rats. J Nucl Med 32: 2266–2272.
Lewis R, Kapur S, Jones C, DaSilva J, Brown GM, Wilson AA et al (1999). Serotonin 5-HT2 receptors in schizophrenia: a PET study using [18F]setoperone in neuroleptic-naive patients and normal subjects. Am J Psychiatry 156: 72–78.
Masellis M, Basile V, Meltzer HY, Lieberman JA, Sevy S, Macciardi FM et al (1998). Serotonin subtype 2 receptor genes and clinical response to clozapine in schizophrenia patients. Neuropsychopharmacology 19: 123–132.
Matsumoto I, Inoue Y, Iwazaki T, Pavey G, Dean B (2005). 5-HT2A and muscarinic receptors in schizophrenia: a postmortem study. Neurosci Lett 379: 164–168.
Meltzer H, Matsubara S, Lee J (1989). Classification of typical and atypical antipsychotic drugs on the basis of dopamine D-1, D-2, and serotonin2 pki values. J Pharmacol Exp Ther 251: 238–246.
Meltzer HY, Li Z, Kaneda Y, Ichikawa J (2003). Serotonin receptors: their key role in drugs to treat schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 27: 1159–1172.
Mita T, Hanada S, Nishino N, Kuno T, Nakai H, Yamadori T et al (1986). Decreased serotonin S2 and increased dopamine D2 receptors in chronic schizophrenics. Biol Psychiatry 21: 1407–1414.
Ngan ET, Yatham LN, Ruth TJ, Liddle PF (2000). Decreased serotonin 2A receptor densities in neuroleptic-naive patients with schizophrenia: a PET study using [(18)F]setoperone. Am J Psychiatry 157: 1016–1018.
Okubo Y, Suhara T, Suzuki K, Kobayashi K, Inoue O, Terasaki O et al (2000). Serotonin 5-HT2 receptors in schizophrenic patients studied by positron emission tomography. Life Sci 66: 2455–2464.
Oquendo MA, Russo SA, Underwood MD, Kassir SA, Ellis SP, Mann JJ et al (2006). Higher postmortem prefrontal 5-HT2A receptor binding correlates with lifetime aggression in suicide. Biol Psychiatry 59: 235–243.
Pinborg LH, Adams KH, Svarer C, Holm S, Hasselbalch SG, Haugbøl S et al (2003). Quantification of 5-HT2A receptors in the human brain using [18F]altanserin–PET and the bolus/infusion approach. J Cereb Blood Flow Metab 23: 985–996.
Pralong D, Tomaskovic-Crook E, Opeskin K, Copolov D, Dean B (2000). Serotonin(2A) receptors are reduced in the planum temporale from subjects with schizophrenia. Schizophr Res 44: 35–45.
Quarantelli M, Berkouk K, Prinster A, Landeau B, Svarer C, Balkay L et al (2004). Integrated software for the analysis of brain PET/SPECT studies with partial-volume-effect correction. J Nucl Med 45: 192–201.
Reynolds GP, Rossor MN, Ivesen LL (1983). Preliminary studies of human cortical 5-HT2 receptors and their involvement in schizophrenia and neuroleptic drug action. J Neural Transm Suppl 18: 273–277.
Skapinakis P, Papatheodorou T, Mavreas V (2007). Antipsychotic augmentation of serotonergic antidepressants in treatment-resistant obsessive–compulsive disorder: a meta-analysis of the randomized controlled trials. Eur Neuropsychopharmacol 17: 79–93.
Sled JG, Zijdenbos AP, Evans AC (1998). A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 17: 87–97.
Svarer C, Madsen K, Hasselbalch SG, Pinborg LH, Haugbøl S, Froekjaer VG et al (2005). MR-based automatic delineation of volumes of interest in human brain PET images using probability maps. NeuroImage 24: 969–979.
Svensson TH, Mathe JM, Andersson JL, Nomikos GG, Hildebrand BE, Marcus M (1995). Mode of action of atypical neuroleptics in relation to the phencyclidine model of schizophrenia: role of 5-HT2 receptor and alpha 1-adrenoceptor antagonism. J Clin Psychopharmacol 15: 11S–18S.
Tan PZ, Baldwin RM, Van Dyck CH, Al-Tikriti M, Roth B, Khan N et al (1999). Characterization of radioactive metabolites of 5-HT2A receptor PET ligand [18F]altanserin in human and rodent. Nucl Med Biol 26: 601–608.
Trichard C, Paillere-Martinot ML, Attar-Levy D, Blin J, Feline A, Martinot JL (1998). No serotonin 5-HT2A receptor density abnormality in the cortex of schizophrenic patients studied with PET. Schizophr Res 31: 13–17.
Verhoeff NP, Meyer JH, Kecojevic A, Hussey D, Lewis R, Tauscher J et al (2000). A voxel-by-voxel analysis of [18F]setoperone PET data shows no substantial serotonin 5-HT(2A) receptor changes in schizophrenia. Psychiatry Res 99: 123–135.
Videbaek C, Friberg L, Holm S, Wammen S, Foged C, Andersen JV et al (1993). Benzodiazepine receptor equilibrium constants for flumazenil and midazolam determined in humans with the single photon emission computer tomography tracer [123I]iomazenil. Eur J Pharmacol 249: 43–51.
Whitaker PM, Crow TJ, Ferrier IN (1981). Tritiated LSD binding in frontal cortex in schizophrenia. Arch Gen Psychiatry 38: 278–280.
Willendrup P, Pinborg LH, Hasselbalch SG, Adams KH, Stahr K, Knudsen GM et al (2004). Assessment of the precision in co-registration of structural MR-images and PET-images with localized binding. Int Congr Ser 275–280. ISBN: 0444515674.
Zhang W, Bymaster FP (1999). The in vivo effects of olanzapine and other antipsychotic agents on receptor occupancy and antagonism of dopamine D1, D2, D3, 5HT2A and muscarinic receptors. Psychopharmacology (Berl) 141: 267–278.
Acknowledgements
The study was sponsored by The Danish Medical Research Council, H:S (Copenhagen Hospital Cooperation) Research Council, Copenhagen University Hospitals Rigshospitalet and H:S Bispebjerg, The John and Birthe Meyer Foundation, The Lundbeck Foundation, and an unrestricted grant from AstraZeneca A/S, Denmark.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure/financial support for the study
The authors have nothing further to disclose.
Rights and permissions
About this article
Cite this article
Erritzoe, D., Rasmussen, H., Kristiansen, K. et al. Cortical and Subcortical 5-HT2A Receptor Binding in Neuroleptic-Naive First-Episode Schizophrenic Patients. Neuropsychopharmacol 33, 2435–2441 (2008). https://doi.org/10.1038/sj.npp.1301656
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1301656
Keywords
This article is cited by
-
Opposite alterations of 5HT2A receptor brain density in subjects with schizophrenia: relevance of radiotracers pharmacological profile
Translational Psychiatry (2021)
-
Measurement of changes in endogenous serotonin level by positron emission tomography with [18F]altanserin
Annals of Nuclear Medicine (2021)
-
Advances in CNS PET: the state-of-the-art for new imaging targets for pathophysiology and drug development
European Journal of Nuclear Medicine and Molecular Imaging (2020)
-
Advances in CNS Imaging Agents: Focus on PET and SPECT Tracers in Experimental and Clinical Use
CNS Drugs (2015)
-
Reduced Levels of Serotonin 2A Receptors Underlie Resistance of Egr3-Deficient Mice to Locomotor Suppression by Clozapine
Neuropsychopharmacology (2012)