Abstract
Neurokinin-3 (NK3) receptors are concentrated in forebrain and basal ganglia structures within the mammalian CNS. This distribution, together with the modulatory influence of NK3 receptors on monoaminergic neurotransmission, has led to the hypothesis that NK3 receptor antagonists may have therapeutic efficacy in the treatment of psychiatric disorders. Here we describe the in vitro and in vivo characterization of the highly selective NK3 receptor antagonist talnetant (SB-223412). Talnetant has high affinity for recombinant human NK3 receptors (pKi 8.7) and demonstrates selectivity over other neurokinin receptors (pKi NK2=6.6 and NK1<4). In native tissue-binding studies, talnetant displayed high affinity for the guinea pig NK3 receptor (pKi 8.5). Functionally, talnetant competitively antagonized neurokinin B (NKB)-induced responses at the human recombinant receptor in both calcium and phosphoinositol second messenger assay systems (pA2 of 8.1 and 7.7, respectively). In guinea pig brain slices, talnetant antagonized NKB-induced increases in neuronal firing in the medial habenula (pKB=7.9) and senktide-induced increases in neuronal firing in the substantia nigra pars compacta (pKB=7.7) with no diminution of maximal agonist efficacy, suggesting competitive antagonism at native NK3 receptors. Talnetant (3–30 mg/kg i.p.) significantly attenuated senktide-induced ‘wet dog shake’ behaviors in the guinea pig in a dose-dependent manner. Microdialysis studies demonstrated that acute administration of talnetant (30 mg/kg i.p.) produced significant increases in extracellular dopamine and norepinephrine in the medial prefrontal cortex and attenuated haloperidol-induced increases in nucleus accumbens dopamine levels in the freely moving guinea pigs. Taken together, these data demonstrate that talnetant is a selective, competitive, brain-penetrant NK3 receptor antagonist with the ability to modulate mesolimbic and mesocortical dopaminergic neurotransmission and hence support its potential therapeutic utility in the treatment of schizophrenia.
Similar content being viewed by others
INTRODUCTION
Tachykinins are a family of structurally related peptide neurotransmitters that share a common amino acid sequence at their C-terminal region. There are five mammalian tachykinins, substance P (SP), neurokinin (NK) A, neurokinin B (NKB), neuropeptide (NP) K and NPγ, which exert their diverse physiological actions via interaction with three family 1 (rhodopsin-like) Gq protein–coupled receptors: NK1, NK2, and NK3. The interaction of the tachykinins with its receptor results in a Gq-mediated activity which ultimately leads to elevation of intracellular Ca2+ via a phospholipase C—IP3/DAG signaling pathway (for review see Maggi, 1995).
The rank order of affinity of tachykinins for the NK3 receptor is NKB>>NKA>SP. Studies of function and localization have demonstrated the presence of NK3 receptors in mammalian central, peripheral, and enteric nervous systems, where their activation influences the release of a variety of neurotransmitters (for review see Maggi, 1995). In the mammalian brain, NK3 receptors are expressed within medial prefrontal cortex (mPFC), thalamus, and amygdala, while their presence in midbrain dopaminergic nuclei (the ventral tegmental area (VTA) and substantia nigra (SN)), locus coeruleus, and septal and basal nuclei suggest a role in modulating central monoaminergic systems (Langlois et al, 2001). However, there are considerable species differences in NK3 receptor expression within the brain (Langlois et al, 2001; Rigby et al, 2005) which can complicate preclinical investigations of this receptor.
Neurochemical studies have shown that NK3 receptor activation can augment dopamine (DA) and acetylcholine (ACh) efflux in the nucleus accumbens (NAcc) and caudate putamen (Marco et al, 1998), norepinephrine (NE) efflux in the mPFC (Jung et al, 1996; Bert et al, 2002) and ACh in the hippocampus (Marco et al, 1998) of the guinea pig, and γ-aminobutyric acid (GABA) in the NAcc and caudate putamen of the rat (Preston et al, 2000). Similarly, electrophysiological data have demonstrated that NK3 receptor activation can augment NE (Jung et al, 1996) and DA cell firing (Nalivaiko et al, 1997) in the guinea pig brain. Furthermore, NK3 receptor antagonists have been shown to attenuate dopaminergic cell firing in the guinea pig VTA and SN, induced by DA D2 receptor and neurotensin receptor blockade (Gueudet et al, 1999).
Taken together, these data suggest a possible role for NK3 receptors in a number of psychiatric diseases and moreover, may indicate that this receptor could be targeted in the treatment of these disorders (for review see Spooren et al, 2005). This hypothesis has recently gained credence with the report that the NK3 receptor antagonist SR-142801 (osanetant) (Meltzer et al, 2004) showed efficacy in schizophrenic patients. Specifically, in a 6-week multicenter, double-blind, placebo-controlled, clinical study in hospitalized schizophrenic and schizoaffective disorder patients osanetant improved four primary efficacy measures, ie the total score from the Positive and Negative Scale for Schizophrenia, the derived score for the Brief Psychiatric Rating Scale (BPRS), the BPRS positive symptom subscale (delusions, hallucinations, conceptual disorganization, and bizarre behavior), and the Clinical Global Impression Scale. Furthermore, the improvement in these patients was not significantly different from that achieved with the antipsychotic haloperidol and the magnitude of the haloperidol-induced improvement was consistent with that observed previously (Meltzer et al, 2004).
We now report the in vitro characterization of the nonpeptide tachykinin receptor antagonist ((S)-(−)-N (α-ethylbenzyl)-3-hydroxy-2-phenylquinoline-4-carboxamide), talnetant (SB-223412; Sarau et al, 1997) at human recombinant and guinea pig native NK3 receptors and present evidence of in vivo activity at brain NK3 receptors. Furthermore, we have investigated the mechanism of action of this compound on central neurochemical function that indicates that this molecule may have therapeutic utility in the treatment of psychiatric disorders.
MATERIALS AND METHODS
Animals
Male Dunkin–Hartley guinea pigs (David Hall or B&K Universal, UK) were housed four per cage in a temperature- (18±2°C) and humidity (55±5%)-controlled environment on a 12 h light/dark cycle with lights on at 0700 hours. Food and water were available ad libitum. All experiments were carried out in accordance with the United Kingdom Animals (Scientific Procedures) Act, 1986 and with GlaxoSmithKline ethical standards.
In Vitro Binding to Human Recombinant and Guinea Pig Native NK3 Receptors
Human cloned NK3 receptors (Sarau et al, 1997) stably expressed in HEK293 cells were harvested at confluence, resuspended in 20 volumes of phosphate-buffered saline (PBS), and then pelleted by centrifugation (1000g/10 min). For preparation of membranes, cell pellets were homogenized (Polytron, 15 s, setting 5) and centrifuged (50 000g, 15 min, 4°C) in 30 volumes of Tris-HCl (50 mM, pH 7.4, 4°C). The membranes were washed with ice-cold buffer, homogenization and centrifuged (50 000g, 12 min, 4°C) three times, and the resultant membranes were finally resuspended at a membrane concentration equivalent to 2 × 107 cells/ml (∼1 mg protein/ml) and stored at −80°C prior to use.
Guinea pig cerebral cortices were dissected and homogenized (Polytron, 15 s, setting 5) in 30 volumes (based on wet weight of tissue) of 50 mM Tris (pH 7.4 at 37°C) and membranes prepared as above.
Recombinant (10–20 μg protein per tube) or cerebral cortex membranes (100–200 μg protein per tube) were incubated in Tris-HCl buffer (50 mM, pH 7.4 at 37°C) containing MgCl2 (10 mM), and bovine serum albumin (BSA, 0.1%) with [3H]SB-222200 (1 nM for competition analysis and eight concentrations within the range 0.1–100 nM for saturation analysis) in the presence or absence of the test compound for 60 min at 37°C. For both saturation and competition analyzes, nonspecific binding was determined using 1 μM SB-787976 (a selective, nonpeptide NK3 receptor antagonist; Blaney et al, 2001). The reaction was terminated by rapid filtration through Whatman GF/B grade filters (presoaked with 0.3% polyethyleneimine) followed by 5 × 1 ml ice-cold buffer washes and bound radioactivity was determined by liquid scintillation counting.
Intracellular Ca2+ Mobilization in HEK293 Cells Stably Expressing the Human NK3 Receptor (hNK3/HEK293)
Changes in intracellular Ca2+ concentrations were monitored using a fluorescence imaging plate reader (FLIPR, Molecular Devices Corp., Sunnyvale, CA, USA) by a method similar to that described by Jerman et al (2001). HEK293 cells were preincubated (37°C for 60 min) with Calcium Plus reagent (Molecular Devices Corp.) in Tyrodes buffer (NaCl, 145 mM; KCl, 2.5 mM; HEPES, 10 mM; glucose, 10 mM; MgCl2, 1.2 mM; CaCl2, 1.5 mM) containing probenecid (2.5 mM) before incubation (30 min, 37°C) with either buffer or antagonist (50 μl). The plates were then placed in the FLIPR, NKB (10 pM–1 μM final concentration) added, and changes in fluorescence monitored.
Inositol Phosphate (IP) Accumulation in U-2OS Cells Transiently Expressing the Human NK3 Receptor
Human osteosarcoma (U-2OS) cells previously transduced with the human NK3 receptor (transduction utilized a recombinant baculovirus under the control of a mammalian promoter generated using vectors and methods described in Ames et al, 2004) were incubated for 16 h with inositol-free DMEM in the presence of [3H]myo-inositol 1 μCi/well (Amersham, UK). The growth medium was then aspirated and the cells washed with 2 × 200 μl inositol-free DMEM/3% BSA. The cells were then preincubated (30 min, 37°C) in the absence or presence of talnetant (1, 3, or 10 nM) and NKB was then added (0.1 nM–10 mM; 30 min, 37°C) in the presence of LiCl (5 mM). In experiments investigating the reversibility of antagonism, a washing stage (3 ×) was included following antagonist incubation and prior to NKB addition. The assay was terminated and cells solubilized by the addition of 0.1 M formic acid (200 μl) and 20 μl aliquots were transferred to Picoplates (Perkin Elmer Life Sciences) containing 80 μl of RNA-Ysi SPA beads (Amersham) previously diluted 1 : 8 (vol:vol) with double deionized water. The Picoplates were shaken for 60 min and then centrifuged (10 min, 1000g) and counted using a scintillation counter.
In Vitro Electrophysiology
Guinea pigs were killed by decapitation and their brains rapidly removed and immersed in an ice-cold sucrose-containing artificial cerebrospinal fluid (aCSF), composed of sucrose 189 mM, glucose 10 mM, NaHCO3 26 mM, KCl 2.5 mM, MgCl2 5 mM, CaCl2 0.1 mM, NaH2PO4 1.2 mM, bubbled continuously with an O2/CO2 mixture (95/5%) to maintain the pH 7.4, 400 μm. Coronal slices containing the dorsal substantia nigra pars compacta (SNpc) or the medial habenula (mHb) were cut using a Vibroslice (Camden Instruments Inc.). Brain slices were subsequently immersed in aCSF containing: NaCl 120 mM, KCl 2.5 mM, CaCl2 1.2 mM, MgCl2 1.5 mMNaHCO3 25 mM, NaHPO4 2.5 mM, glucose 10 mM, again bubbled with 95/5% O2/CO2, for at least 30 min prior to initiation of recording. A single slice was then transferred to a recording chamber (submerged configuration) that was continuously perfused with oxygenated aCSF at a rate of 2–4 ml/min and maintained at 33°C.
Extracellular recordings were obtained using microelectrodes (3–5 MΩ; filled with aCSF) placed in the SNpc or mHb under visual control. Presumptive dopaminergic neurons in the SNpc were identified by a low-frequency (∼2 Hz) regular firing pattern and reversible depression of spontaneous activity in response to application of the DA receptor agonist quinpirole (3 μM; Nalivaiko et al, 1997). Neurons from the mHb were identified as those which exhibited spontaneous activity in the range of 2–8 Hz and which displayed an increase in activity in response to 1 min applications of 300 nM NKB (Boden and Woodruff, 1994). Only neurons that met the criteria for their respective nuclei were selected for further analysis. Electrical signals were fed into a high-input impedance preamplifier (AxoClamp 2B, Axon Instruments Inc.), amplifier (Brownlee), and digitizer (Axon Instruments Inc.). Baseline activity was recorded for 5–10 min before drugs were applied via Tyvek tubing connected to the recording chamber, allowing complete exchange of fluids within 2 min of the arrival of a new solution. Senktide (3 nM–1 μM) was bath applied for 5–8 min, until a steady-state effect was reached, with a 15–20 min washout between concentrations. NKB (10 nM–10 μM) was applied for 1 min to minimize desensitization, with 10–20 min washout between concentrations. Antagonists were pre-applied for 30 min.
Autoradiographical Determination of Cortical Ex Vivo Receptor Occupancy
Guinea pigs were administered vehicle (1% methylcellulose, 2 ml/kg) or talnetant (1, 3, 10, 30, or 100 mg/kg i.p., n=4 per group). After 1 h of dose administration, animals were killed and brains were rapidly removed and frozen in isopentane at approximately −40°C. Hindbrain and trunk blood samples were collected and analyzed for drug concentration (see below). Subsequently, 14 μm thick coronal sections, from between 15.4 and 15.8 mm anterior to the frontal zero plane as defined by Rapisarda and Bacchelli (1977), were cut using a Leica CM3050 cryostat and thaw-mounted onto Superfrost Plus slides (BDH) then stored at −80°C until use.
Slides were thawed and rapidly dried under a stream of cold air then subjected to a 10 min incubation at room temperature with 10 nM [3H]senktide (Perkin Elmer; 51.9 Ci/mmol) in 50 mM Tris-HCl (pH 7.5) containing 3 mM MnCl2, 0.02% (w/v) BSA (Roche), 40 μg/ml bacitracin (Sigma), 2 μg/ml chymostatin (Sigma), and 4 μg/ml leupeptin (Roche). Adjacent sections were incubated in the same solution with the addition of 10 μM SR-142801 to define nonspecific binding. Slides were then subjected to a series of four short (1 min) washes in ice-cold 50 mM Tris-HCl (pH 7.5) followed by two rinses in ice-cold deionized water and were then rapidly dried under a stream of cold air.
Sections were analyzed by direct β-imaging (Beta Imager, BioSpace, Paris) for 12 h and bound radioactivity was measured (counts per minute per square millimetre) in the mPFC using Beta Vision Plus software (BioSpace).
Blood and brain samples and standards were extracted by protein precipitation by addition of ‘precipitation buffer’ (95 : 5 MeCN: EtOH containing 0.1% formic acid and an internal standard) followed by centrifugation at 3000 r.p.m. for 15 min. The resulting supernatant (100 μL) was removed and analyzed for talnetant using LC-MS-MS.
Chromatographic separations were performed using gradient HPLC on Acquity UPLC BEH C18 columns (50 × 2.1 mm) and Waters Acquity UPLC (Waters, Mildford, USA). Separation was achieve via a gradient (mobile phase A=5% MeCN/95% water/0.1% formic acid, mobile phase B=95% MeCN/5% Water/0.1% formic acid) transition from 85 : 15 to 5 : 95% over 1.1 min at a flow rate of 0.5 ml/min. Talnetant was detected using Waters Micromass Quattro Premier VAA301 mass spectrometer (Waters) and quantified using Masslynx v 4.0 software for the PC.
Guinea Pig ‘Wet Dog Shake’ Behavior
Guinea pigs were anesthetized with a 3% isoflurane and 0.5 l/min oxygen mixture. A unilateral intracerebroventricular (i.c.v.) cannula (5 mm length, 22 G; coordinates AP=+9.8; L=±2.3; DV=−4.0 from intra-aural line and skull surface) was directed at the lateral ventricle and fixed in position using three tether screws and cyanoacrylate adhesive. Animals were allowed 7 days' post-operative care prior to experimentation.
Prior to testing, guinea pigs were handled daily and habituated to clear plastic observation cages on at least five occasions. On test days, guinea pigs were administered senktide (0.3 μg, i.c.v.) (Bachem, UK) and 180 s later wet dog shake (WDS) behavior was scored for 30 min by trained observers who were blind to treatment group. Senktide was dissolved in 20 mM acetic acid (0.1%)/PBS solution and administered using an injection needle extending 1 mm beyond the tip of the i.c.v. cannula. Senktide or vehicle solution was injected in a volume of 5 μl. Talnetant (3, 10, or 30 mg/kg, n=9–10) or vehicle (1% methyl cellulose in water) was administered by i.p. injection 60 min prior to senktide administration.
Microdialysis
Guinea pigs were anesthetized by gaseous administration of isofluorane. Microdialysis guide cannulae (CMA 11, CMA, UK) were implanted for sampling from either the NAcc or mPFC and dorsal hippocampus (dHipp). Implantation coordinates were measured from bregma (AP and ML) and the surface of the dura (DV) and are listed in Table 1. Guide cannulae were secured with dental cement and tether anchor screw (Instech, Presearch, Hitchin, UK) attached. Animals were allowed 7 days' postoperative care prior to experimentation.
Microdialysis was performed according to the methods described by Hughes and Dawson (2004). In brief, microdialysis probes (2 mm cuprophane membrane, CMA11 14/02, CMA) were implanted and perfused with aCSF containing NaCl 145 mM, KCl 2.7 mM, MgCl2 1.0 mM, CaCl2 1.2 mM, Na2HPO4 2.0 mM at 1 μl/min. After 2 h of equilibration, four basal microdialysis samples were collected. Animals were simultaneously implanted with mPFC and dHipp probes then received either vehicle or talnetant (30 mg/kg, i.p.) or the positive comparator clozapine (10 mg/kg, s.c.). Guinea pigs implanted with NAcc probes were initially dosed (1 ml/kg, i.p.) with a pretreatment of vehicle (1% methylcellulose) or talnetant (10 or 30 mg/kg) followed 30 min later with either haloperidol (0.5 mg/kg, i.p.) or vehicle. A 30 min sampling regime was used throughout and all microdialysate samples were collected and analyzed for DA, NE and 5-HT content. At the end of the experiment, probes were removed and animals returned to their home cages. Animals were reused in a randomized crossover design with 7 days between each use and on a maximum of four occasions (Hughes and Dawson, 2004). After the final microdialysis experiment, animals were killed and their brains were removed and stored in formalin before verification of probe placement. Brains were sectioned (50 μm) using a cooled cryostat and sections stained with cresyl violet to visualize sites of probe implantation. Data from animals with incorrect probe placement were discarded from the analysis.
Chromatographic separations were performed using a Capcell PAK, strong cation exchange column (5 μm UG80, 1.5 × 150 mm; Shiseido, Tokyo, Japan). The mobile phase, consisted of NaCl 5 mM, Na2HPO4 13 mM, NaH2PO4 87 mM, EDTA.Na2 0.1 mM, in 20% methanol buffered to pH 6 was delivered via a Jasco PU-980 HPLC pump (Jasco, Tokyo, Japan) at a flow rate of 0.2 ml/min at a temperature of 40°C. DA, NE, and 5-HT were detected via an electrochemical amperometric detector (Decade, Antec-Leyden, Leyden, The Netherlands) fitted with a 3 mm glassy carbon electrode set a +500 mV vs Ag/AgCl reference. The analog data output was smoothed at 40 Hz (LINK, Antec-Leyden) before collection. Samples (10 μl) were injected via a cooled (4°C) Gilson model 234 autosampler (Gilson, Villiers-le-Bel, France) fitted with a six-port rotary valve (Model 7125, Rheodyne, Berkeley, CA, USA) with a 20 μl injection loop. All data were acquired using Millenium32 software (Waters).
Data Analysis
The concentration of drug which inhibited the specific binding of [3H]SB-222200 by 50% (IC50) was derived using a four-parameter logistic equation (GraphPad Prism, GraphPad Software Inc.). pKi values (−log of the inhibition constant) were then calculated from the IC50 values as described by Cheng and Prusoff (1973). Data are expressed as the mean±SEM of at least three separate experiments each performed using duplicate determinations.
In FLIPR experiments, peak changes in fluorescence occurred within the first 5 s and were reported following baseline subtraction. Concentration–response curves were analyzed using a four-parameter logistic equation (GraphPad Prism, GraphPad Software Inc.) to obtain pEC50 values (−log EC50). A pA2 value for talnetant was obtained by Schild analysis (Schild, 1947). Data are expressed as mean±SEM of three separate experiments.
Extracellular recordings of action potentials were collected and analyzed using pClamp 9.0 software (Axon Instruments Inc.) or Spike 2 (Cambridge Electronic Design, UK). The effect of senktide was evaluated by measuring the mean action potential discharge frequency during the final 2 min of exposure to each concentration of drug, when a steady-state effect had been reached. In the case of NKB, no steady state was reached, so the 60 s period of maximal activity was used to assess drug effects. Neuronal firing frequency in the presence of senktide was expressed as a percentage of baseline (pre-drug). NKB effects were normalized to the baseline (pre-drug) response to 300 nM NKB. Concentration–response curves were fitted and IC50 values calculated using Origin 5.0 (Microcal) or Prism 4 (Graphpad). pKB values were calculated using the Gaddum equation (Gaddum, 1937).
Data for total number of WDS are expressed as mean±SEM. Data were analyzed by one-way ANOVA followed by Dunnet's t-test.
For occupancy determinations, specific binding was determined as the mean of bilateral measurements from two sections per animal (non-specific binding) subtracted from the mean of bilateral measurements from four sections per animal (total binding). Percent occupancy values were calculated using the following formula: ((vehicle group mean-specific binding−individual animal-specific binding)/vehicle group mean-specific binding) × 100. Curve fitting was carried out using one-site binding nonlinear regression in Prism v4.02.
In microdialysis experiments, the mean concentration of neurotransmitter in the first four baseline samples was calculated and this value denoted as 100%. Values for all samples were expressed as a percentage of this mean preinjection control value. These transformed data were analyzed by two-way ANOVA with repeated measures followed by post hoc Fisher's test where appropriate. Postdrug administration of total DA efflux was calculated as area under the curve using a trapezoidal calculation. These transformed data were analyzed by one-way ANOVA with repeated measures followed by Fisher's least squares differences test.
RESULTS
Saturation Analysis of [3H]SB-222200 Binding to Human-Cloned NK3 Receptors
SB-222200 has previously been reported to be a potent NK3 receptor antagonist (Sarau et al, 2000) and to displace [125I][MePhe7] NKB binding to the human-cloned receptor with a Ki of 4.4 nM. SB-222200 was tritiated (48 Ci/mmol, Tocris, UK) and used to radiolabel the human-cloned and native NK3 receptor from guinea pig. The NK3 receptor affinity for talnetant was determined from [3H]SB-222200 competition binding. Talnetant displayed high affinity for both the human (pKi 8.7±0.10) and guinea pig (pKi 8.5±0.06) NK3 receptors.
Selectivity Screening
As previously reported (Sarau et al, 1997) talnetant is >100-fold selective for the NK3 receptor vs the other members of the tachykinin receptor subfamily. Furthermore, talnetant was cross-screened at concentrations of 1 or 10 μM and was without affinity/effect at 68 enzymes, ion channels, and receptors includingPDE1−4, PKC, PLA2, ionotropic glutamate receptors, L-type Ca2+ channels, adenosine (A1, A2) receptors, serotonin (5-HT1A, 5-HT1D, 5-HT1E, 5-HT2A, 5-HT2C, 5-HT4) receptors, DA (D1, D2, D3, D4) receptors, histamine (H1, H2) receptors, muscarinic (M1, M2) receptors, GABAA or GABAB receptors, and opiod receptor. Although not specifically examined for talnetant, all subsequent quinoline-like NK3 receptor antagonists (the chemical class to which talnetant belongs; Sarau et al, 1997; Blaney et al, 2001) have shown no affinity for DA, serotonin or NE transporters, or NE receptors (α1, α2) (data not shown).
Intracellular Ca2+ Mobilization Using FLIPR
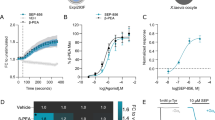
Talnetant showed no evidence of agonist activity at concentrations up to 300 nM (data not shown). However, talnetant (30 nM–1 μM) produced a parallel rightward shift of the NKB concentration–response curve that was surmountable at all concentrations tested (Figure 1). Schild analysis of the data gave a pA2 of 8.20±0.2 and a slope of 0.94±0.09 which was not significantly different from unity. Talnetant, therefore, showed a profile consistent with potent competitive NK3 receptor antagonist activity in this assay system.
The effect of talnetant (30 nM–1 μM) on the neurokinin B (NKB)-stimulated increase in (Ca2+)i in HEK293 cells stably expressing the human NK3 receptor. Data points represent the peak increase in fluorescence and are the mean±SEM from at least three separate experiments and are shown as the percentage of the fitted maximal response to NKB. Inlay shows Schild plot of these data.
Inositol Phosphate Accumulation in U-2OS Cells Transiently Expressing the Human NK3 Receptor
Talnetant (0.1–1 μM) attenuated the NKB-induced accumulation of IP in hNK3/U-2OS cells producing a rightward shift of the NKB concentration–response curve, again with no effect on the agonist-induced maximal response. Schild analysis of the data gave a pA2 of 7.7±0.3. Repeated (× 3) cell washes (following talnetant incubation and prior to agonist application) completely reversed the antagonist effect of talnetant suggesting that the antagonism by talnetant was reversible (Figure 2).
The effect of talnetant (0.1–10 μM) on the neurokinin B (NKB)-induced IP accumulation in U-2OS cells transiently expressing the human NK3 receptor. (a) Effect of talnetant on NKB responses and (b) effects of talnetant following a wash procedure prior to NKB addition. Data points are the mean±SEM from three separate experiments each performed using duplicate determinations.
Effects of Talnetant on NKB-Induced Neuronal Firing in the Medial Habenula
Under control conditions, the mean (±SEM) firing rate of NKB-sensitive neurons in the mHb was 4.4±0.4 Hz (n=11). NKB (10 nM–3 μM) produced a concentration-dependent increase in neuronal firing with an EC50 of 91 nM (n=6; Figure 3a). In a separate set of recordings, application of 100 nM talnetant resulted in a nonsignificant reduction in spontaneous firing from 3.4±0.3 to 2.6±0.5 Hz (n=5, p=0.08, paired t-test). In the presence of 100 nM talnetant, there was a rightward shift in the concentration–response curve with no significant effect on the maximum agonist efficacy (n=5, p=0.93, F-test). The EC50 of NKB under these conditions was 788 nM, resulting in an apparent pKB for talnetant of 7.9 (Figure 3a).
The effect of talnetant on the mean concentration-dependent increase in medial habenula (mHb) neuronal firing elicited by neurokinin B (NKB) (a) and substantia nigra pars compacta (SNpc) neuronal firing elicited by senktide (b) in the guinea pig slice. Under control conditions and in the presence of talnetant (100 nM) that yielded respective EC50 values of 91 nM (n=6) and 788 nM (n=5) in the mHb. Under control conditions and in the presence of talnetant (100 nM) that yielded EC50 values of 26 (n=15) and 140 nM (n=3) in the SNpc. Mean neuronal firing frequencies are expressed as a mean±SEM percentage of baseline (predrug).
Effects of Talnetant on Senktide-Induced Dopamine Neuronal Firing in the Substantia Nigra
Under control conditions, the mean (±SEM) firing rate of presumptive dopaminergic SNpc neurons was 2.2±0. 8 Hz (n=27). Senktide (1 nM–1 μM) produced a concentration-dependent increase in neuronal firing yielding an EC50 of 26 nM (n=15) (Figure 3b). In separate recordings, the concentration–response curve of senktide was determined in the presence of talnetant (100 nM) following a 45 min preincubation with the antagonist (n=3). Application of talnetant (100 nM) to the bath perfusion medium was without effect on baseline neuronal firing. In the presence of talnetant, a parallel shift in the senktide concentration–response curve was observed with no change in maximum response and yielding an EC50 for senktide of 140 nM and an apparent pKB for talnetant of 7.4 (Figure 3b).
Occupancy of Guinea Pig Cortical NK3 Receptors
As demonstrated by the dose-dependent decrease in specific binding of the NK3-specific radioligand [3H]senktide (Figure 4a), i.p. administration of talnetant produced dose-dependent occupancy of NK3 receptors in guinea pig mPFC (Figure 4b) with a calculated ID50 of 3.3 mg/kg. Concurrent measurement of concentrations of talnetant within the whole brain showed that this correlated with an IC50 of 9.6 ng/g and that concentrations above 100 ng/g produce maximal occupancy (Figure 4c).
Relationship between ex vivo cortical NK3 receptor occupancy and dose of talnetant administered and subsequent concentration measured in brain. (a) [3H]senktide ex vivo binding to brain sections from guinea pigs treated with vehicle, 1, 10, or 100 mg/kg talnetant. (b) NK3 receptor occupancy (mean±SEM, n=6 per group) by talnetant in guinea pig mPFC determined by ex vivo [3H]senktide autoradiography. (c) The relationship between NK3 receptor occupancy and concentration of talnetant in brain samples from the same experimental animals. Each point represents data from an individual animal.
Effects of Talnetant on Senktide-Induced Guinea Pig WDS Behaviors
Talnetant (30 mg/kg i.p.) alone produced a small but significant reduction in baseline behaviors (from 2.4±0.5 to 0.6±0.3; p<0.05; Figure 5). The NK3 receptor-selective agonist senktide significantly increased WDS behavior from 2.4±0.5 to 31.1±5.1 (p<0.05; Figure 5). Talnetant (3–30 mg/kg i.p.) produced a significant (F5, 51 46.6, p<0.05) attenuation of this senktide-induced WDS behavior at 30 mg/kg only (from 31.1±5.1 to 16.7±4.1; p<0.05; Figure 5).
Effects of Talnetant on Extracellular Levels of DA, NE, and 5-HT in the mPFC and dHipp
Mean (±SEM) basal levels (pM) of DA, NE, and 5-HT in the guinea pig mPFC were 289.2±27.0 (n=27), 313.6±15.0 (n=31), and 196.0±11.7 (n=29), respectively. Talnetant (30 mg/kg, i.p.) produced a significant increase in extracellular levels of DA, reaching a maximum level of 149.4±22% of preinjection control levels at t=180 min (F1, 12=10.5; p<0.01). Similarly, NE increased to a maximum of 165.4±14% of preinjection control levels at t=30 min (F1, 12=8.6; p<0.05) in the mPFC (Figure 6). Similarly, the positive comparator clozapine (10 mg/kg, s.c.) produced significant (F1, 15=13.4; p<0.005 and F1, 15=19.3; p<0.001) increases in both neurotransmitters (maximum increases: DA, 238±65%, t=120 min; NE, 227.1±42%, t=60 min) (Figure 6). No changes were observed in extracellular levels of 5-HT with either drug treatment (data not shown).
Comparative effects of talnetant and clozapine on dopamine (DA) and norepinephrine (NE) efflux in prefrontal cortex (PFC) and hippocampus of the guinea pig. Data expressed as mean±SEM (n=8–12 per group). **p<0.01, ***p<0.001 vs vehicle. Note that basal levels of DA in the hippocampus were not consistently above the limits of detection of the analytical systems used, thus data on this transmitter have not been presented.
Mean basal levels (pM) of NE and 5-HT in the guinea pig dHipp were 319.2±19.3 (n=29) and 255.5±18.8 (n=30), respectively. Basal levels of DA were below the limits of consistent detection in this brain region and as such data have not been included. Talnetant (30 mg/kg, i.p.) produced a significant (F1, 12=23.3, p<0.001) increase in extracellular levels of NE that reached a maximum of 204.3±32% of preinjection control levels while having no effect on extracellular 5-HT (Figure 6). Similarly, clozapine (10 mg/kg, s.c.) produced a significant (F1, 14=46.7, p<0.0001) increase in extracellular NE (maximum increases: 224.9±28%, t=90 min) but produced no change in 5-HT (data not shown).
Effects of Talnetant on Haloperidol-Induced Increases in NAcc Extracellular Levels of Dopamine: In Vivo Microdialysis
Basal levels of DA in the guinea pig NAcc were 1071±78 pM (n=32). Repeated measures ANOVA revealed a significant overall effect of treatment for the interaction study between talnetant and haloperidol (F4, 91=7.7, p<0.0001; Figure 7). Post hoc analysis revealed no significant effect of talnetant (30 mg/kg, i.p.) alone. Haloperidol (0.5 mg/kg, s.c.) produced a significant (p<0.0001) increase in extracellular levels of DA reaching a maximum value of 202.2±18.5% (t=150 min). Haloperidol-induced increases were significantly (p=0.0002) attenuated by 30 mg/kg of talnetant. A similar trend was also seen at 10 mg/kg of talnetant but this failed to reach statistical significance (p=0.16).
DISCUSSION
Here we describe the in vitro and in vivo characterization of the highly selective NK3 receptor antagonist talnetant (SB-223412; Sarau et al, 1997) and provide evidence in support of this hypothesis that NK3 receptors may be useful as a therapeutic target for the treatment of psychotic disorders.
Talnetant exhibits high affinity for the human NK3 receptor as determined using antagonist radioligand ([3H]SB-222200) binding. This value (pKi 8.7) was not substantially different from that previously reported using the endogenous agonist radioligand NKB (pKi 9.0; Sarau et al, 1997). Cross-species native tissue binding, using the same antagonist radioligand, revealed that the affinity of talnetant for the guinea pig cortical NK3 receptor (pKi 8.5) was very similar to that at the human receptor. Differences in the pharmacological properties of peptidomimetic small molecule ligands for tachykinin receptors across species is well documented (for review see Maggi, 1995) and other NK3 receptor antagonist ligands, such as PD-154740, PD-157672 (Maggi, 1995), and SR-142801 (Chung et al, 1995), have all been demonstrated to have a much reduced affinity for rodent vs human receptors. The implications of interspecies variation for preclinical models will be discussed later.
Both Ca2+ imaging and IP accumulation assays were used to probe the functional consequences of NK3 receptor ligands on the Gq signaling cascade, although it could be argued that because the IP assay permits both agonist (NKB) and antagonist to attain an equilibrium status that this assay environment is more akin to the physiological situation. In both assay systems, talnetant was revealed to be a high affinity NK3 receptor antagonist that produced a concentration-dependent parallel rightward shift in the NKB concentration–response curve with no appreciable decrease in the excitatory maximum response (Emax), suggestive of a competitive mechanism of action. Furthermore, antagonism was lost following a washout procedure, demonstrating a reversible activity. Thus, talnetant is a competitive, reversible antagonist at the recombinant human NK3 receptor in vitro.
The functional activity of talnetant was further evaluated in native tissue preparations. Since the affinity of talnetant for the guinea pig NK3 receptor appears to be similar to the human homolog, we employed electrophysiological recordings in guinea pig brain slices. Neurons within the guinea pig mHb have previously been described as having a high sensitivity to NK3 receptor agonists and an apparent absence of NK1 and NK2 receptor-mediated responses which makes this nucleus ideal for studies of central NK3-mediated events (Boden and Woodruff, 1994). Talnetant produced a parallel rightward shift in the NKB-induced neuronal excitations with no appreciable decrease in Emax, again indicative of competitive antagonist activity, in this case in a native tissue preparation.
Wet dog shake is a form of stereotypical behavior that may be induced in guinea pigs through the i.c.v. administration of the potent peptide NK3 receptor agonist senktide (Renzetti et al, 1991). This behavior has been previously shown to be blocked by the NK3 receptor antagonist SR-142801 (Yip and Chahl, 1997) and we now show that systemic administration of talnetant produced significant attenuation, thereby demonstrating the central blockade of NK3 receptors at a 30 mg/kg dose. Based on our ex vivo occupancy studies, systemic administration of talnetant to guinea pigs also resulted in a dose- and brain concentration-dependent increase in occupancy of mPFC NK3 receptors (as measured by displacement of the agonist radioligand senktide; Langlois et al, 2001) with a 30 mg/kg dose generating mean total brain concentrations of talnetant of 111 ng/g and effectively achieving maximal NK3 receptor occupancy.
The DA hypothesis of schizophrenia has guided some aspects of schizophrenia research for the past four decades. Although the hypothesis has gained in complexity, and to a degree is now yielding to a multifactorial hypothesis involving monoamines, glutamate and GABA, the underlying hyperfunctionality of the mesolimbic DA system and a hypofrontality are still believed to be core facets of schizophrenia pathology (for review see Carlsson et al, 2001). Thus, direct or indirect modulation of forebrain dopaminergic systems provides a valid focus for research into the development of novel therapeutics for the disease. The expression of NK3 receptors in ventral mesencephalic DA cells and their apparent localization on dopaminergic cell bodies (Dam et al, 1990; Stoessl, 1994; Chen et al, 1998) was the first indication that these tachykinin receptors may be potential modulators of dopaminergic neurotransmission. Biochemical and electrophysiological studies with tachykinin receptor agonists provided supportive evidence (Humpel et al, 1991; Keegan et al, 1992; Bannon et al, 1986; Alonso et al, 1996) and the more recent development of selective, small molecule NK3 receptor antagonists has confirmed this hypothesis. In this regard, the NK3 receptor agonists senktide and the endogenous ligand NKB were demonstrated to stimulate dopaminergic cell firing in guinea pig slice preparations (Nalivaiko et al, 1997). These responses were blocked by the NK3 receptor antagonist SR-142801 but not NK1 or NK2 receptor antagonists. Using in vivo microdialysis techniques in guinea pigs, local administration of senktide into dopaminergic cell bodies of the mesencephalic and SNpc resulted in an enhanced DA output in terminal projection regions such as mPFC, NAcc, and striatum. Again, these responses were blocked by SR-142801 confirming the NK3 receptor-mediated dopaminergic responses (Marco et al, 1998).
Using in vitro electrophysiological recordings of dopaminergic cell firing in the guinea pig SNpc, we demonstrated that talnetant produced a parallel rightward shift in the senktide-induced excitations with no appreciable decrease in Emax. This response is, again, indicative of the competitive receptor antagonist activity of talnetant in this native tissue assay. The pKB was 7.4, which was in line with the data in the mHb (pKB 7.9). These values are, however, somewhat lower than those generated in vitro at the human receptor. As the binding affinity for the guinea pig receptor is similar to that at the human NK3 receptor, this discrepancy is likely to be the result of incomplete equilibrium of talnetant within the slice. Nevertheless, these observations do confirm that talnetant can block NK3 receptors that are presumably located on dopaminergic cell bodies of the SNpc. To determine if these receptors play a role in modulating dopaminergic neurotransmission in vivo, we investigated the impact of talnetant on DA efflux in a region receiving afferent projections from the midbrain dopaminergic nuclei. The NAcc (separation of core and shell substructures of the NAcc is not feasible due to the poor anatomical differentiation in the guinea pig (Rapisarda and Bacchelli, 1977)) receives mixed innervation from both the SNpc and VTAs, and is implicated in the hypothesized hyperfunction of the mesolimbic projection systems in schizophrenia. Talnetant alone had no effect on basal DA efflux. This observation is consistent with previous in vivo studies, utilizing both microdialysis (Marco et al, 1998) and electrophysiology (Gueudet et al, 1999), which demonstrated that SR-142801 was without effect when administered alone. Taken together, these data suggest that there is no detectable tonic activation of NK3 receptors on dopaminergic cell bodies in the guinea pig under these conditions. SNpc/VTA cell firing can be increased by acute administration of haloperidol, an effect that could be attenuated with SR-142801 (Gueudet et al, 1999). We have shown that haloperidol increased extracellular DA efflux in the NAcc, presumably via blockade of the tonically active DA D2 autoreceptor, and that pretreatment with talnetant produced a dose-related attenuation of the haloperidol-induced increase in extracellular DA. These data confirm and extend the findings of Gueudet et al (1999) that NK3 receptor antagonism can reduce hyperactivity in dopaminergic cell firing and consequently attenuate a hyperactive mesolimbic DA output. The reason that the NK3-mediated depression of DA efflux in the NAcc is revealed in the presence of haloperidol may simply be that blockade of the primary dopaminergic D2 autoreceptor increases the activity of the dopaminergic nuclei (Gueudet et al, 1999) revealing the NKB-mediated drive on the system. Interestingly, both the haloperidol-induced increases in DA efflux in the present study and the neuronal firing data (Gueudet et al, 1999) appear to be only partially reversed by NK3 receptor antagonism. The simplest explanation for this is that a proportion of the haloperidol-induced response is mediated through removal of tonic negative-feedback circuitry, mediated by endogenous DA acting at the D2 autoreceptor. As NK3 receptor antagonists do not modulate basal firing or DA levels per se, they are, thus, unlikely to fully reverse the D2 receptor-mediated effects of haloperidol.
NK3 receptors have also been shown to have modulatory actions on other neurotransmitter systems including NE (Jung et al, 1996; Bert et al, 2002) and 5-HT (Liu et al, 2002). Thus, talnetant was examined for its ability to modulate efflux of NE, 5-HT, and DA in the mPFC and dHipp. Previous data have suggested that direct activation of NK3 receptors, via application of senktide either directly to cell bodies (Marco et al, 1998; Bert et al, 2002; Liu et al, 2002) or i.c.v. (Jung et al, 1996), resulted in an increase in extracellular levels of NE, DA, and 5-HT in cortical structures. It was therefore somewhat surprising that talnetant induced increases in mPFC DA and NE, and enhanced dHipp NE levels. Notably, these changes were similar to those induced by the atypical antipsychotic clozapine (which has no reported affinity for NK3 receptors) in the same study. However, unlike clozapine, talnetant possesses no affinity for the monoaminergic sites of action (ie D1−4, 5-HT2, 5-HT1 etc) generally associated with this class of antipsychotics.
These findings indicate that there is a tonic activation of NK3 receptors that impact upon dopaminergic and noradrenergic projections to cortical and hippocampal structures. Based on published studies (Marco et al, 1998; Bert et al, 2002) and our own observations, the populations of NK3 receptors that mediate this effect are not likely to be on the cell bodies of the locus coeruleus or midbrain dopaminergic nuclei, since administration of NK3 receptor agonists directly to these areas also enhanced output of monoamines in the cerebral cortex and hippocampus. NK3 receptors have been demonstrated to regulate GABAergic neurotransmission (Preston et al, 2000) and colocalization studies indicate that NK3 receptors are expressed on GABAergic interneurons of basal ganglia nuclei including striatum, globus pallidus, ventral pallidum, and substantia inominata (Preston et al, 2000; Furuta et al, 2004). Furthermore, subpopulations of basal ganglia GABA neurons appear to send projections to the cerebral cortex (Furuta et al, 2004). Interestingly, a proportion of these GABAergic neurons are parvalbumin positive and this subpopulation of interneurons has been implicated in the pathophysiology of schizophrenia (for review see Lewis et al, 2005). Therefore, one possible explanation for talnetant-induced stimulation of monoamine efflux in the cerebral cortex and hippocampus is that NK3 receptors, localized on GABAergic interneurons, are tonically active under normal physiological conditions and exert a tonic inhibitory action on basal ganglion and cortical neurotransmission. Thus, antagonism of these populations of NK3 receptors disinhibits basal ganglia GABAergic neurotransmission thereby enhancing cortical dopaminergic and noradrenergic neurotransmission. Further work is required to establish to confirm this hypothesis.
Interspecies variation in NK3 receptor location and function adds to the complexity of unraveling NK3 receptor biology. NK3 receptors are distributed in the medial and deep-layer structures of the cortex, hypothalamus, and midbrain dopaminergic structures such as the SN and VTA of the rat. In the guinea pig distribution in mid- and deep-layer cortical structures is largely similar to the rat but differs in the midbrain dopaminergic neurons where NK3 receptors have not been detected (Yip and Chahl, 2001). However, NK3-mediated functional changes in these nuclei, measured using electrophysiological recordings and subsequent neurochemical output, have been demonstrated (Navilaiko et al, 1997; Marco et al, 1998). PCR (Buell et al, 1992) and, more recently, immunocytochemistry techniques (Mileusnic et al, 1999; Koutcherov et al, 2000) have established that NK3 receptors are also expressed, albeit in low abundance, in the primate CNS and human distribution studies have shown that the deep-layer cortical distribution appears to be maintained (Tooney et al, 2000). However, no NK3 receptors have been detected, to date, within the mesencephalic dopaminergic neurons (Dietl and Palacios, 1991; Whitty et al, 1994, 1997). Thus, in addition to the complications of using preclinical species to evaluate the functional role of the NK3 receptors, there is also some controversy with regard to the specific localization and density of NK3 receptors within the human brain (Rigby et al, 2005). Although, in this regard our in house studies, using autoradiographic and immunohistochemical techniques, have detected widespread but low-level expression of the NK3 receptors within human brain (unpublished data). Despite these remaining uncertainties, the hypothesis that NK3 receptor antagonists have antipsychotic activity (Spooren et al, 2005) has gained support following preliminary reports suggesting that the NK3 receptor antagonists SR-142801 (osanetant; Meltzer et al, 2004) may be clinically efficacious. The ability of NK3 receptor antagonists to attenuate dopaminergic cell firing (Gueudet et al, 1999) and the present demonstration of reduced DA output from the mesolimbic system in the guinea pig may provide a mechanism which accounts for the amelioration of positive symptoms (PANNS and BPRS) reported in the SR-142801 trial. Additionally, the enhancement of guinea pig prefrontal and hippocampal monoaminergic neurotransmission may also suggest the potential to modulate the hypofrontality associated with schizophrenia (Carlsson et al, 2001). Thus, the neurochemical profile of talnetant is suggestive of potential therapeutic activity in the treatment of multiple symptom domains of schizophrenia and we await the outcome of further clinical trials with interest.
In conclusion, talnetant is a competitive antagonist at both the human and guinea pig NK3 receptors. In vivo, the systemic administration of talnetant produced occupancy and antagonism of central NK3 receptors. Dopaminergic hyperactivity in the mesolimbic projection systems was stimulated by NK3 receptor activation in the guinea pig SNpc and attenuated by talnetant. Furthermore, talnetant blocked tonically active NK3 receptors resulting in enhanced noradrenergic and dopaminergic neurotransmission in forebrain structures producing a neurochemical profile similar to the atypical antipsychotic clozapine. The present data offer support for the hypothesis that NK3 receptor antagonists may be efficacious in the treatment of schizophrenia.
References
Alonso R, Fournier M, Carayon P, Petitpretre G, Le Fur G, Soubrie P (1996). Evidence for the modulation of dopamine-neuronal function by tachykinin NK3 receptor stimulation in gerbils. Eur J Neurosci 8: 801–808.
Ames R, Fornwald J, Nuthulaganti P, Trill J, Foley J, Buckley P et al (2004). BacMam recombinant baculoviruses in G protein-coupled receptor drug discovery. Receptors Channels 10: 99–107.
Bannon MJ, Lee J-M, Giraud P, Young A, Affolter HU, Bonner TI (1986). The dopamine antagonist haloperidol decreases substance P, substance K, and preprotachykinin mRNAs in rat striatonigral neurons. J Biol Chem 261: 6640–6642.
Bert L, Rodier D, Bougault I, Allouard N, Le Fur G, Soubrie P et al (2002). Permissive role of neurokinin NK3 receptors in NK1 receptor-mediated activation of the locus coeruleus revealed by SR-142801. Synapse 43: 62–69.
Blaney FE, Raveglia LF, Artico M, Cavagnera S, Dartois C, Farina C et al (2001). Stepwise modulation of neurokinin-3 and neurokinin-2 receptor affinity and selectivity in quinoline tachykinin receptor antagonists. J Med Chem 44: 1675–1689.
Boden P, Woodruff GN (1994). Presence of NK3-sensitive neurones in different proportions in the medial habenula of guinea-pig, rat and gerbil. Br J Pharmacol 112: 717–719.
Buell G, Schulz MF, Arkinstall SJ, Maury K, Missotten M, Adami N et al (1992). Molecular characterisation, expression and localisation of human neurokinin-3 receptor. FEBS Lett 299: 90–95.
Carlsson A, Waters N, Holm-Waters S, Tedroff J, Nilsson M, Carlsson M (2001). Interactions between monoamines, glutamate and GABA in schizophrenia: new evidence. Annu Rev Pharmacol Toxicol 41: 237–260.
Chen LW, Guan ZL, Ding YQ (1998). Mesencephalic dopaminergic neurons expressing neuromedin K receptor (NK3): a double immunocytochemical study in the rat. Brain Res 780: 148–152.
Cheng Y, Prusoff WH (1973). Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50 per cent inhibition (IC50) of an enzymatic reaction. Biochem Pharmacol 22: 3099–3108.
Chung FZ, Wu LH, Tian Y, Vartanian MA, Lee H, Bikker J et al (1995). Two classes of structurally different antagonists display similar species preference for the human tachykinin neurokinin3 receptor. Mol Pharmacol 48: 711–716.
Dam TV, Escher E, Quirion R (1990). Visualization of neurokinin-3 receptor sites in rat brain using the highly selective ligand [3H]senktide. Brain Res 506: 175–179.
Dietl MM, Palacios JM (1991). Phylogeny of tachykinin receptor localization in the vertebrate central nervous system: apparent absence of neurokinin-2 and neurokinin-3 binding sites in the human brain. Brain Res 539: 211–222.
Furuta T, Koyano K, Tomioka R, Yanagawa Y, Kaneko T (2004). GABAergic basal forebrain neurons that express receptor for neurokinin B and send axons to the cerebral cortex. J Comp Neurol 473: 43–58.
Gaddum JH (1937). The quantitative effects of antagonistic drugs. J Physiol 89: 7P–9P.
Gueudet C, Santucci V, Soubrie P, Le Fur G (1999). Blockade of neurokinin3 receptors antagonizes drug-induced population response and depolarization block of midbrain dopamine neurons in guinea pigs. Synapse 33: 71–79.
Hughes ZA, Dawson LA (2004). Differential autoreceptor control of extracellular 5-HT in guinea pig and rat: species and regional differences. Psychopharmacology 172: 87–93.
Humpel C, Saria A, Regoli D (1991). Injection of tachykinins and selective neurokinin receptor ligands into the substantia-nigra reticulata increases striatal dopamine and 5-hydroxytryptamine metabolism. Eur J Pharmacol 195: 107–114.
Jerman JC, Brough SJ, Gager T, Wood M, Coldwell MC, Smart D et al (2001). Pharmacological characterisation of human 5-HT2 receptor subtypes. Eu J Pharmacol 414: 23–30.
Jung M, Michaud JC, Steinberg R, Barnouin MC, Hayar A, Mons G et al (1996). Electrophysiological, behavioural and biochemical evidence for activation of brain noradrenergic systems following neurokinin NK3 receptor stimulation. Neuroscience 74: 403–414.
Keegan KD, Woodruff GN, Pinnock RD (1992). The selective NK3 agonist senktide excites a subpopulation of dopamine-sensitive neurones in the rat substantia nigra pars compacta in vitro. Br J Pharmacol 105: 3–5.
Koutcherov Y, Ashwell KW, Paxinos G (2000). The distribution of the neurokinin B receptor in the human and rat hypothalamus. Neuroreport 11: 3127–3131.
Langlois X, Wintmolders C, te Riele P, Leyson JE, Jurzak M (2001). Detailed distribution of neurokinin 3 receptors in the rat, guinea pig and gerbil brain: a comparative autoradiography study. Neuropharmacology 40: 242–253.
Lewis DA, Hashimoto T, Volk DW (2005). Cortical inhibitory neurons and schizophrenia. Nature Rev Neurosci 6: 312–324.
Liu R, Ding Y, Aghajanian GK (2002). Neurokinins activate local glutamatergic inputs to serotonergic neurons of the dorsal raphe nucleus. Neuropsychopharmacology 27: 329–340.
Maggi CA (1995). The mammalian tachykinin receptors. Gen Pharmacol 26: 911–944.
Marco N, Thirion A, Mons G, Bougault I, Le Fur G, Soubrie R et al (1998). Activation of dopaminergic and cholinergic neurotransmission by tachykinin NK3 receptor stimulation: an in vivo microdialysis approach in guinea pig. Neuropeptides 32: 481–488.
Meltzer HY, Arvanitis L, Bauer D, Rein W (2004). Placebo-controlled evaluation of four novel compounds for the treatment of schizophrenia and schizoaffective disorder. Am J Psychiatry 161: 975–984.
Mileusnic D, Lee JM, Magnuson DJ, Hejna MJ, Krause JE, Lorens JB et al (1999). Neurokinin-3 receptor distribution in rat and human brain: an immunohistochemical study. Neuroscience 89: 1269–1290.
Nalivaiko E, Michaud J-C, Soubrie P, Le Fur G, Feltz P (1997). Tachykinin neurokinin1 and neurokinin3 receptor mediated responses in guinea pig substantia nigra: in vitro electrophysiological study. Neuroscience 78: 745–757.
Preston Z, Richardson PJ, Pinnock RD, Lee K (2000). NK3 receptors are expressed on mouse striatal gamma-aminobutyric acid-ergic interneurones and evoke [3H] gamma-aminobutyric acid release. Neurosci Letts 284: 89–92.
Rapisarda C, Bacchelli B (1977). The brain of the guinea pig in stereotaxic coordinates. Arch Sci Biol 61: 1–37.
Renzetti AR, Barsacchi P, Criscuoli M, Lucacchini A (1991). Characterization of NK3 binding sites in rat and guinea pig cortical membranes by the selective ligand [3H]senktide. Neuropeptides 18: 107–114.
Rigby M, O'Donnell R, Rupniak NM (2005). Species differences in tachykinin receptor distribution: further evidence that the substance P (NK1) receptor predominates in human brain. J Comp Neurol 490: 335–353.
Sarau HM, Griswold DE, Bush B, Potts W, Sandhu P, Lundberg D et al (2000). Non-peptide tachykinin receptor antagonists II. Pharmacological and pharmacokinetic profile of SB-222200 a central nervous system penetrant, potent and selective NK-3 receptor antagonist. J Pharmacol Exp Ther 295: 373–381.
Sarau HM, Griswold DE, Potts W, Foley JJ, Schmidt DB, Webb EF et al (1997). Nonpeptide tachykinin receptor antagonists: I. Pharmacological and pharmacokinetic characterization of SB 223412, a novel, potent and selective neurokinin-3 receptor antagonist. J Pharmacol Exp Ther 281: 1303–1311.
Schild HO (1947). pA, a new scale for the measurement of drug antagonism. Br J Pharmacol 2: 189–206.
Spooren W, Riemer C, Meltzer H (2005). Opinion: NK3 receptor antagonists: the next generation of antipsychotics? Nat Rev Drug Discov 4: 967–975.
Stoessl AJ (1994). Localization of striatal and nigral tachykinin receptors in the rat. Brain Res 646: 13–18.
Tooney PA, Au GG, Chahl LA (2000). Tachykinin NK1 and NK3 receptors in the prefrontal cortex of the human brain. Clin Exp Pharm Phys 27: 947–949.
Whitty CJ, Paul MA, Bannon MJ (1997). Neurokinin receptor mRNA localisation in human midbrain dopamine neurons. J Comp Neurol 382: 394–400.
Whitty CJ, Walker PD, Goebel DJ, Poosch MS, Bannon MJ (1994). Quantitation, cellular localization and regulation of neurokinin receptor gene expression within the substantia nigra. Neuroscience 64: 419–425.
Yip J, Chahl LA (1997). Localization of Fos-like immunoreactivity induced by the NK3 tachykinin receptor agonist, senktide, in the guinea-pig brain. Br J Pharmacol 122: 715–725.
Yip J, Chahl LA (2001). Localization of NK1 and NK3 receptors in guinea-pig brain. Regul Pept 98: 55–62.
Author information
Authors and Affiliations
Corresponding author
Additional information
AUTHORSHIP DISCLOSURE
All authors are employees of GlaxoSmithKline Plc.
Rights and permissions
About this article
Cite this article
Dawson, L., Cato, K., Scott, C. et al. In Vitro and In Vivo Characterization of the Non-peptide NK3 Receptor Antagonist SB-223412 (Talnetant): Potential Therapeutic Utility in the Treatment of Schizophrenia. Neuropsychopharmacol 33, 1642–1652 (2008). https://doi.org/10.1038/sj.npp.1301549
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1301549