Abstract
The present study was undertaken to examine whether the second generation antibiotic drug minocycline attenuates behavioral changes (eg, acute hyperlocomotion and prepulse inhibition (PPI) deficits) in mice after the administration of the N-methyl-D-aspartate (NMDA) receptor antagonist (+)-MK-801 (dizocilpine). Dizocilpine (0.1 mg/kg)-induced hyperlocomotion was significantly attenuated by pretreatment with minocycline (40 mg/kg). Furthermore, the PPI deficits after a single administration of dizocilpine (0.1 mg/kg) were attenuated by pretreatment with minocycline (10, 20, or 40 mg/kg), in a dose-dependent manner. Moreover, in vivo microdialysis study in the free-moving mice revealed that pretreatment with minocycline (40 mg/kg, i.p.) significantly attenuated the increase of extracellular dopamine (DA) levels in the frontal cortex and striatum after administration of dizocilpine (0.1 mg/kg), suggesting that the inhibition of dizocilpine-induced DA release by minocycline may, at least in part, be implicated in the mechanism of action of minocycline with respect to dizocilpine-induced behavioral changes in mice. These findings suggest that minocycline could attenuate behavioral changes in mice after the administration of the NMDA receptor antagonist dizocilpine. Therefore, it is possible that minocycline would be a potential therapeutic drug for schizophrenia.
Similar content being viewed by others
INTRODUCTION
Multiple lines of evidence suggest that a dysfunction in the glutamatergic neurotransmission via the N-methyl-D-aspartate (NMDA) receptors might be involved in the pathophysiology of schizophrenia (Javitt and Zukin, 1991; Olney and Farber, 1995; Coyle, 1996; Krystal et al, 1999; Tamminga, 1998; Hashimoto et al, 2003, 2004, 2005). Therefore, the NMDA receptor antagonists such as (+)-MK-801 (dizocilpine) have been used widely in animal models for schizophrenia (Al-Amin and Schwarzkopf, 1996; Hashimoto et al, 1997; Bakshi and Geyer, 1998; Varty et al, 1999; Morimoto et al, 2002; Okamura et al, 2004).
Prepulse inhibition (PPI) of the acoustic startle response is a form of sensorimotor gating, defined as an inhibition of the startle response when a low-intensity stimulus, the prepulse, precedes the startling stimulus (Braff and Geyer, 1990; Braff and Freedman, 2002; Geyer et al, 2001). Deficits in PPI have been reported in several psychiatric disorders including schizophrenia, suggesting that deficient PPI per se or abnormalities in neural circuits regulating PPI may cause some symptoms (eg, cognitive deficits) of schizophrenia (Braff and Geyer, 1990; Perry et al, 1999; Swerdlow and Geyer, 1998; Braff and Freedman, 2002). In experimental animals, PPI deficits can be induced by the administration of the NMDA receptor antagonist dizocilpine (Al-Amin and Schwarzkopf, 1996; Bakshi and Geyer, 1998; Varty et al, 1999; Yee et al, 2004; Long et al, 2006). Therefore, pharmacological models of PPI deficits by NMDA receptor antagonism are excellent predictors of antipsychotic activity (Swerdlow and Geyer, 1998; Geyer et al, 2001; Levin et al, 2005).
Accumulating evidence suggest that the second-generation tetracycline minocycline produces neuroprotective effects in several animal models of neurological diseases, including Parkinson's disease (Du et al, 2001; Wu et al, 2002), amyotrophic lateral sclerosis (Zhu et al, 2002), Huntington's disease (Chen et al, 2000; Wang et al, 2003), and ischemia (Yrjanheikki et al, 1998, 1999). The neuroprotective effects of minocycline can occur indirectly by microglial activation and proliferation (Yrjanheikki et al, 1998; Tikka et al, 2001; Wu et al, 2002; Domercq and Matute, 2004; Yong et al, 2004; Blum et al, 2004; Thomas and Le, 2004; Stirling et al, 2005). Recently, we reported that minocycline could ameliorate the behavioral changes (eg, acute hyperlocomotion and the development of behavioral sensitization) and neurotoxicity in mice or monkey by the administration of methamphetamine or 3,4-methylenedioxymethamphetamine (Zhang et al, 2006a, 2006b; Hashimoto et al, 2007), suggesting that minocycline may be a potential therapeutic drug for neuropsychiatric disorders including schizophrenia.
In this study, we investigated the effects of minocycline on behavioral changes (acute hyperlocomotion and PPI deficits) in mice induced by the administration of dizocilpine. Furthermore, using in vivo microdialysis technique, we examined the effects of minocycline on the dopamine (DA) release in prefrontal cortex and striatum after the administration of dizocilpine as DA in these brain regions has been implicated in the behavioral changes by the NMDA receptor antagonism.
METHODS
Animals
Male Std:ddy mice (8 weeks old, 32–39 g body weight at the beginning of the experiment) were housed under a 12-h light/12-h dark cycle (lights on from 0700 to 1900 h; room temperature, 22±2°C; humidity, 55±5%) with free access to food and water. All experiments were performed in accordance with the Guide for Animal Experimentation, Chiba University Graduate School of Medicine.
Drugs Administration
(+)-MK-801 hydrogen maleate (dizocilpine) (0.1 mg/kg. as a hydrogen maleate salt; Sigma-Aldrich Corporation, St Louis, MO), dissolved in physiological saline, was injected subcutaneously (s.c.) in a volume of 10 ml/kg. The dose (0.1 mg/kg) of dizocilpine was selected because this dose caused PPI deficits in mice. Minocycline hydrochloride (10, 20, or 40 mg/kg as a hydrochloride salt; Wako Pure Chemical Industries, Ltd, Osaka, Japan), dissolved in physiological saline, was injected intraperitoneally (i.p.) in a volume of 10 ml/kg. The other chemicals used were purchased from commercial sources.
Effects of Minocycline on Hyperlocomotion after a Single Administration of Dizocilpine
Thirty minutes after a single i.p. injection of minocycline (10, 20, or 40 mg/kg, n=6) or vehicle (10 ml/kg, n=6), dizocilpine (0.1 mg/kg, n=6) or vehicle (10 ml/kg, n=6) was administered s.c. into the mice. Locomotor activity was measured using an animal movement analysis system (SCANET SV-10, Melquest, Toyama, Japan), as reported previously (Zhang et al, 2006a). The system consisted of a rectangular enclosure (480 × 300 mm). The side walls (height, 60 mm) of the enclosure were equipped with 144 pairs of photosensors located at 5-mm intervals at a height of 30 mm from the bottom edge. An animal was placed in the observation cage 60 min from injection of vehicle or dizocilpine. A pair of photosensors was scanned every 0.1 s to detect the animal's movements. The intersection of paired photosensors (10 mm apart) in the enclosure was counted as one unit of locomotor activity. Data collected for 180 min were used in this study.
Measurement of Acoustic Startle Reactivity and Prepulse Inhibition of Startle
The mice were tested for their acoustic startle reactivity (ASR) in a startle chamber (SR-LAB, San Diego Instruments, CA) using standard methods described by Swerdlow and Geyer (1998). After an initial 10-min acclimation period in the chamber, the test sessions began. They consisted of six trial types: (1) pulse alone, 40 ms broadband burst; pulse preceded 100 ms by a 20 ms prepulse that was (2) 4 dB, (3) 8 dB, (4) 12 dB, or (5) 16 dB over background (65 dB); and (6) background only (no stimulus). The amount of PPI is expressed as the percentage decrease in the amplitude of the startle reactivity caused by presentation of the prepulse (% PPI).
For the effect of minocycline on PPI, minocycline (10, 20, or 40 mg/kg) or vehicle (10 ml/kg) were administered 40 min (including 10-min acclimation period) before the machine records, and dizocilpine (0.1 mg/kg) or vehicle (10 ml/kg) was administered s.c. 10 min (including 10-min acclimation period) before. The PPI test lasted 20 min in total.
In Vivo Microdialysis
Mice were anesthetized with sodium pentobarbital before the stereotaxic implantation of a probe into the left frontal cortex (+2.1 mm anteroposterior, +1.0 mm mediolateral from the bregma, and −1.2 mm dorsoventral with respect to dura) or striatum (+0.0 mm anteroposterior, +2.5 mm mediolateral from the bregma, and −4.4 mm dorsoventral with respect to dura). Probes were secured onto the skull using stainless-steel screws and dental acrylic. Twenty-four hours after surgery, in vivo microdialysis was performed on conscious mice. Probes were perfused continuously with artificial CSF (147 mM NaCl, 4 mM KCl, and 2.3 mM CaCl2) at a rate of 2 μl/min. The dialysate was collected in 30-min fractions. Levels of DA were measured by high-performance liquid chromatography (HPLC) using a reversed phase column (Eicompak CA-5ODS 2.1 mm × 150 mm; Eicom, Kyoto, Japan), as reported previously (Zhang et al, 2006a). Four samples were obtained in order to establish the baseline levels of extracellular DA before the administration of dizocilpine.
Statistical Analysis
The data are presented as the mean±standard error of the mean (SEM). The computation was carried out using the SPSS 12.0J software (SPSS 12.0J, Tokyo, Japan). The results of the acute behavioral study and in vivo microdialysis were analyzed by two-way analysis of variance (ANOVA) for repeated measures, with treatment as the between-subjects factor and time as the within-subjects factor. When appropriate, group means at individual time points were compared by one-way ANOVA, followed by Bonferroni/Dunn a posteriori analysis.
PPI was calculated as the percent inhibition of the startle amplitude evoked by the pulse alone: % PPI=100 × (magnitude on pulse alone trial−magnitude on prepulse+pulse trial/magnitude on pulse alone trial). The PPI data were analyzed using a with treatment drug as a between-subjects factor and prepulse intensity as a within-subjects factor. There were significant effects of prepulse intensity (which were always significant), which will not be discussed, and drug treatment data were collapsed across prepulse intensity for presentation purposes. The PPI data were analyzed by multivariate analysis of variance (MANOVA). When appropriate, group means at individual dB levels were compared by one-way ANOVA, followed by Bonferroni/Dunn a posteriori analysis. The dose-dependent relationship was evaluated by MANOVA, followed by one-way ANOVA with contrast (polynomial). Significance for the results was set at p<0.05.
RESULTS
Effects of Minocycline on Hyperlocomotion after a Single Administration of Dizocilpine
A single administration of dizocilpine (0.1 mg/kg, s.c.) markedly increased locomotion in mice. Two-way ANOVA analysis revealed significant differences among the five groups studied (F(44, 275)=2.599, p<0.0001). Pretreatment with minocycline (40 mg/kg, i.p., 30 min before the administration of dizocilpine) significantly attenuated dizocilpine-induced hyperlocomotion in mice (Figure 1). In contrast, administration of minocycline (40 mg/kg) alone did not alter locomotion in mice.
Effects of minocycline on dizocilpine-induced hyperlocomotion in mice. Thirty minutes after a single i.p. injection of minocycline (10, 20, or 40 mg/kg) or vehicle (10 ml/kg), dizocilpine (0.1 mg/kg) or vehicle (10 ml/kg) was administered s.c. into the mice. Behavior (locomotion) in the mice was evaluated. Each value (counts per 10 min) is the mean±SEM (n=6 per group). *p<0.05, **p<0.01 as compared with the vehicle+dizocilpine group.
Effects of Minocycline on PPI Deficits after a Single Administration of Dizocilpine
Figure 2 shows the effects of minocycline (10, 20, or 40 mg/kg) on dizocilpine (0.1 mg/kg)-induced PPI deficits in mice. The MANOVA analysis of all PPI data revealed that there was a significant effect (Wilks lambda=0.395, p<0.001). Subsequent ANOVA analysis revealed significant differences at all dB groups (4, 8, 12, and 16 dB). A posteriori analysis indicated a significant (p<0.01) difference between vehicle+vehicle group and vehicle+dizocilpine (0.1 mg/kg) group (Figure 2). Furthermore, a posteriori analysis demonstrated that minocycline (40 mg/kg) significantly (p<0.05) attenuated PPI deficits in mice induced by dizocilpine (0.1 mg/kg) (Figure 2). Next, we analyzed whether the effects of minocycline on dizocilpine-induced PPI deficits were dose-dependent. The MANOVA analysis of four groups (0, 10, 20, and 40 mg/kg of minocycline) revealed a significance (Wilks lambda=0.621, p=0.029). Moreover, the subsequent analysis using contrast (polynomial) showed that minocycline significantly attenuated dizocilpine-induced PPI deficits at 8 dB (p=0.003), 12 dB (p<0.001), and 16 dB (p<0.001), in a dose-dependent manner (Figure 2). In contrast, minocycline (40 mg/kg) alone did not alter PPI in mice (Figure 2).
The effect of minocycline on dizocilpine-induced PPI deficits in mice. Thirty minutes after i.p. injection of vehicle (10 ml/kg) or minocycline (10, 20, or 40 mg/kg), dizocilpine (0.1 mg/kg) or vehicle (10 ml/kg) was administered s.c. to the mice. Each value is the mean±SEM (n=12–14 per group). **p<0.01 as compared with vehicle+vehicle group, #p<0.05 as compared with vehicle+dizocilpine group.
Effects of Minocycline on Dizocilpine-Induced DA Release in the Frontal Cortex and Striatum
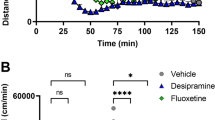
In order to explore the mechanisms by which minocycline inhibits the psychopharmacological effects of dizocilpine, we used an in vivo microdialysis technique to examine the in vivo effects of minocycline on the dizocilpine-induced increase in extracellular DA levels in the frontal cortex and striatum of conscious mice. A single administration of dizocilpine (0.1 mg/kg, s.c.) caused a marked increase in extracellular DA levels in the frontal cortex and striatum. Peak levels of extracellular DA were increased to approximately five-fold the baseline level. Two-way ANOVA analysis revealed significant differences among the three groups studied (frontal cortex: F(10, 110)=58.47, p<0.001; striatum: F(10, 100)=60.07, p<0.001). Subsequent analysis revealed that pretreatment with minocycline (40 mg/kg, i.p., 30 min before dizocilpine treatment) significantly attenuated dizocilpine-induced increases in extracellular DA levels in the frontal cortex (Figure 3a) and in the striatum (Figure 3b). Effects of minocycline on dizocilpine-induced DA release in the frontal cortex were greater than those of minocycline in the striatum. In contrast, we found that minocycline alone did not alter the extracellular DA levels in the frontal cortex (Figure 3a) and striatum (Figure 3b).
Effects of minocycline on extracellular DA levels in the frontal cortex and striatum after the administration of dizocilpine. Thirty minutes after i.p. injection of minocycline (40 mg/kg) or vehicle (10 ml/kg), MK-801 (0.1 mg/kg, s.c.) or vehicle (10 ml/kg, s.c.) was administered to mice. Extracellular levels of DA in the mouse frontal cortex (a) and striatum (b) were measured by in vivo microdialysis in conscious mice. The basal extracellular DA levels were 0.424±0.019 pg/20 μl in the frontal cortex (mean±SEM of 8–9 mice) and 2.697±0.269 pg/20 μl in the striatum (mean±SEM of 8–9 mice). *p<0.05, **p<0.01 compared with dizocilpine-treated group.
DISCUSSION
The major findings of the present study are that minocycline significantly attenuated behavioral changes (hyperlocomotion and PPI deficits) in mice after the administration of dizocilpine, and that minocycline significantly attenuated increase of extracellular DA levels in the frontal cortex and striatum after the administration of dizocilpine. To our knowledge, this is the first report demonstrating that minocycline can restore behavioral changes (eg, hyperlocomotion and sensorimotor gating deficits) induced by the NMDA receptor antagonist dizocilpine. Several studies demonstrated that atypical antipsychotic drugs including clozapine can ameliorate hyperlocomotion and PPI deficits in mice after the administration of dizocilpine (Leriche et al, 2003; Levin et al, 2005; Lipina et al, 2005; Long et al, 2006). Therefore, our findings indicate that minocycline has a potential antipsychotic activity in animal models of schizophrenia.
Schizophrenia is associated with a dysregulation of DA function in both the prefrontal cortex and striatum (reviewed by Goldman-Rakic, 1999; Goldman-Rakic et al, 2004; Weinberger et al, 2001; Abi-Dargham and Moore, 2003), and the role of prefrontal cortex in working memory had received a great deal of attention because most patients with schizophrenia exhibit deficits in working memory-related tasks (reviewed by Goldman-Rakic, 1999; Goldman-Rakic et al, 2004). It has been reported that the NMDA receptor antagonists such as dizocilpine and ketamine dose-dependently impaired the spatial delayed alteration performance, and that these drugs preferentially increased the release of DA in the prefrontal cortex compared with the striatum of rats (Verma and Moghaddam, 1996). Interestingly, it has been reported that repeated administration of dizocilpine significantly increased the density of DA D1 receptors in the prefrontal cortex and decreased working memory performance in monkeys (Tsukada et al, 2005), indicating the dizocilpine-induced impairment of DA neuronal system in prefrontal cortex. A recent report showed that DA D1 receptor agonists rather than D2 receptor agonists disrupt PPI in mice, suggesting that DA D1 receptors may play a more prominent role in the modulation of PPI in mice (Ralph-Williams et al, 2003). Taken together, it is likely that the inhibition of dizocilpine-induced DA release by minocycline in the prefrontal cortex may be implicated in the mechanism of action of minocycline with respect to dizocilpine-induced PPI deficits in mice although the mechanism(s) underlying the modulation of dizocilpine-induced DA release by minocycline are currently unclear. Therefore, it is likely that minocycline may have potential therapeutic activity for schizophrenia.
Some studies demonstrated that the medial prefrontal cortex (mPFC) might be involved in the PPI deficits after the administration of dizocilpine (Bakshi and Geyer, 1998; Schwabe and Koch, 2004). First, it has been reported that dizocilpine significantly decreased PPI after infusion into the amygdala or dorsal hippocampus, but not nucleus accumbens, ventral hippocampus, or dorsomedial thalamus, and that a trend toward PPI deficits was also observed with administration into mPFC (Bakshi and Geyer, 1998). These findings suggest that multiple limbic forebrain regions including mPFC might mediate dizocilpine-induced PPI deficits in rats (Bakshi and Geyer, 1998). Second, Schwabe and Koch (2004) reported that dizocilpine failed to disrupt PPI in rats with ibotenic acid lesions of the mPFC, suggesting that mPFC is an important brain region within the neuronal circuit responsible for dizocilpine-induced PPI deficits. In this study, we found that the increase in extracellular DA levels in prefrontal cortex after the administration of dizocilpine was significantly attenuated by pretreatment with minocycline (40 mg/kg). Based on the key role of DA in the behavioral changes by the NMDA receptor antagonists, it is also likely that the inhibition of dizocilpine-induced DA release by minocycline in the prefrontal cortex may, in part, be implicated in the mechanism of action of minocycline with respect to dizocilpine-induced behavioral changes in mice.
Minocycline can readily cross the blood–brain barrier, regardless of the dose and route of administration (Barza et al, 1975; Aronson, 1980; Zhang et al, 2006a). Recent clinical trials have been aimed primarily at assessing the safety and tolerability of minocycline in several neurodegenerative diseases (reviewed by Blum et al, 2004; Domercq and Matute, 2004; Thomas and Le, 2004; Yong et al, 2004; Stirling et al, 2005; Smith and Leyden, 2005). In these clinical trials, minocycline was well tolerated at 200 mg/day over 6 months, and no side effects or negative interactions with other simultaneously administered drugs were observed (Domercq and Matute, 2004). Taken together, it might be of great interest to study the effects of minocycline on several symptoms in schizophrenic patients.
In conclusion, the present findings suggest that minocycline ameliorated behavioral changes (hyperlocomotion and PPI deficits) in mice after the administration of the NMDA receptor antagonist dizocilpine, and minocycline significantly attenuated the release of DA in the frontal cortex after the administration of dizocilpine. Therefore, minocycline would be a potential therapeutic drug for schizophrenia.
References
Abi-Dargham A, Moore H (2003). Prefrontal DA transmission at D1 receptors and the pathology of schizophrenia. Neuroscientist 9: 404–416.
Al-Amin HA, Schwarzkopf SB (1996). Effects of the PCP analog dizocilpine on sensory gating: potential relevance to clinical subtypes of schizophrenia. Biol Psychiatry 40: 744–754.
Aronson AL (1980). Pharmacotherapeutics of the newer tetracyclines. J Am Vet Med Assoc 176: 1061–1067.
Bakshi VP, Geyer MA (1998). Multiple limbic regions mediate the disruption of prepulse inhibition produced in rats by the noncompetitive NMDA antagonist dizocilpine. J Neurosci 18: 8394–8401.
Barza M, Brown RB, Shanks C, Gamble C, Weinstein L (1975). Relation between lipophilicity and pharmacological behavior of minocycline, doxycycline, tetracycline, and oxytetracycline in dogs. Antimicrob Agents Chemother 8: 713–720.
Blum D, Chtarto A, Tenenbaum L, Brotchi J, Levivier M (2004). Clinical potential of minocycline for neurodegenerative disorders. Neurobiol Dis 17: 359–366.
Braff DL, Freedman R (2002). Endophenotypes in studies of the genetics of schizophrenia. In: Davis KL, Charney D, Coyle JT, Nemeroff C (eds). Neuropsychopharmacology—The Fifth Generation of Progress. Lippincott Williams & Wilkins: Philadelphia, PA. pp 703–716.
Braff DL, Geyer MA (1990). Sensorimotor gating and schizophrenia. Human and animal model studies. Arch Gen Psychiatry 47: 181–188.
Chen M, Ona VO, Li M, Ferrante RJ, Fink KB, Zhu S et al (2000). Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat Med 7: 797–801.
Coyle JT (1996). The glutamatergic dysfunction hypothesis for schizophrenia. Harv Rev Psychiatry 3: 241–253.
Domercq M, Matute C (2004). Neuroprotection by tetracyclines. Trends Pharmacol Sci 25: 609–612.
Du Y, Ma Z, Lin S, Dodel RC, Gao F, Bales KR et al (2001). Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson's disease. Proc Natl Acad Sci USA 98: 14669–14674.
Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR (2001). Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 156: 117–154.
Goldman-Rakic PS (1999). The physiological approach: functional architecture of working memory and disordered cognition in schizophrenia. Biol Psychiatry 46: 650–661.
Goldman-Rakic PS, Castner SA, Svensson TH, Siever LJ, Williams GV (2004). Targeting the dopamine D1 receptor in schizophrenia: insights for cognitive dysfunction. Psychopharmacology (Berl) 174: 3–16.
Hashimoto K, Fukushima T, Shimizu E, Komatsu N, Watanabe H, Shinoda N et al (2003). Decreased serum levels of D-serine in patients with schizophrenia: evidence in support of the N-methyl-D-aspartate receptor hypofunction hypothesis of schizophrenia. Arch Gen Psychiatry 60: 572–576.
Hashimoto K, Okamura N, Shimizu E, Iyo M (2004). Glutamate hypothesis of schizophrenia and approach for possible therapeutic drugs. Curr Med Chem CNS Agents 4: 147–154.
Hashimoto K, Shimizu E, Iyo M (2005). Dysfunction of glia–neuron communication in pathophysiology of schizophrenia. Curr Psychiatry Rev 1: 151–163.
Hashimoto K, Tomitaka S, Bi Y, Narita N, Minabe Y, Iyo M (1997). Rolipram, a selective phosphodiesterase type-IV inhibitor, prevents induction of heat shock protein HSP-70 and hsp-70 mRNA in rat retrosplenial cortex by the NMDA receptor antagonist dizocilpine. Eur J Neurosci 9: 1891–1901.
Hashimoto K, Tsukada H, Nishiyama S, Fukumoto D, Kakiuchi T, Iyo M (2007). Protective effects of minocycline on the reduction of dopamine transporters in the striatum after administration of methamphetamine: a PET study in conscious monkeys. Biol Psychiatry, in press.
Javitt DC, Zukin SR (1991). Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry 148: 1301–1308.
Krystal JH, D'Souza DC, Petrakis IL, Belger A, Berman RM, Charney DS et al (1999). NMDA agonists and antagonists as probes of glutamatergic dysfunction and pharmacotherapies in neuropsychiatric disorders. Harv Rev Psychiatry 7: 125–143.
Leriche L, Schwartz JC, Sokoloff P (2003). The dopamine D3 receptor mediates locomotor hyperactivity induced by NMDA receptor blockade. Neuropharmacology 45: 174–181.
Levin ED, Petro A, Caldwell DP (2005). Nicotine and clozapine actions on pre-pulse inhibition deficits caused by N-methyl-D-aspartate (NMDA) glutamatergic receptor blockade. Prog Neuropsychopharmacol Biol Psychiatry 29: 581–586.
Lipina T, Labrie V, Weiner I, Roder J (2005). Modulators of the glycine site on NMDA receptors, D-serine and ALX 5407, display similar beneficial effects to clozapine in mouse models of schizophrenia. Psychopharmacology (Berl) 179: 54–67.
Long LE, Malone DT, Taylor DA (2006). Cannabidiol reverses MK-801-induced disruption of prepulse inhibition in mice. Neuropsychopharmacology 31: 795–803.
Morimoto T, Hashimoto K, Yasumatsu H, Tanaka H, Fujimura M, Kuriyama M et al (2002). Neuropharmacological profile of a novel potential atypical antipsychotic drug Y-931 (8-fluoro-12-(4-methylpiperazin-1-yl)- 6H-[1]benzothieno[2,3-b][1,5] benzodiazepine maleate). Neuropsychopharmacology 26: 456–467.
Okamura N, Hashimoto K, Shimizu E, Kumakiri C, Komatsu N, Iyo M (2004). Adenosine A1 receptor agonists block the neuropathological changes in rat retrosplenial cortex after administration of the NMDA receptor antagonist dizocilpine. Neuropsychopharmacology 29: 544–550.
Olney JW, Farber NB (1995). Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry 52: 998–1007.
Perry W, Geyer MA, Braff DL (1999). Sensorimotor gating and thought disturbance measured in close temporal proximity in schizophrenic patients. Arch Gen Psychiatry 56: 277–281.
Ralph-Williams RJ, Lehmann-Masten V, Geyer MA (2003). Dopamine D1 rather than D2 receptor agonists disrupt prepulse inhibition of startle in mice. Neuropsychopharmacology 28: 108–118.
Schwabe K, Koch M (2004). Role of the medial prefrontal cortex in N-methyl-D-aspartate receptor antagonist induced sensorimotor gating deficit in rats. Neurosci Lett 355: 5–8.
Smith K, Leyden JJ (2005). Safety of doxycycline and minocycline: a systematic review. Clin Ther 27: 1329–1342.
Stirling DP, Koochesfahani KM, Steeves JD, Tetzlaff W (2005). Minocycline as a neuroprotective agent. Neuroscientist 11: 308–322.
Swerdlow NR, Geyer MA (1998). Using an animal model of deficient sensorimotor gating to study the pathophysiology and new treatments of schizophrenia. Schizophr Bull 24: 285–301.
Tamminga CA (1998). Schizophrenia and glutamatergic transmission. Crit Rev Neurobiol 12: 21–36.
Thomas M, Le WD (2004). Minocycline: neuroprotective mechanisms in Parkinson's disease. Curr Pharm Des 10: 679–686.
Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J (2001). Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci 21: 2580–2588.
Tsukada H, Miyasato K, Nishiyama S, Fukumoto D, Kakiuchi T, Domino EF (2005). Nicotine normalizes increased prefrontal cortical dopamine D1 receptor binding and decreased working memory performance produced by repeated pretreatment with MK-801: a PET study in conscious monkeys. Neuropsychopharmacology 30: 2144–2153.
Varty GB, Bakshi VP, Geyer MA (1999). M100907, a serotonin 5-HT2A receptor antagonist and putative antipsychotic, blocks dizocilpine-induced prepulse inhibition deficits in Sprague–Dawley and Wistar rats. Neuropsychopharmacology 20: 311–321.
Verma A, Moghaddam B (1996). NMDA receptor antagonists impair prefrontal cortex function as assessed via spatial delayed alternation performance in rats: modulation by dopamine. J Neurosci 16: 373–379.
Wang X, Zhu S, Drozda M, Zhang W, Stavrovskaya IG, Cattaneo E et al (2003). Minocycline inhibits caspase-independent and -dependent mitochondrial cell death pathways in models of Huntington's disease. Proc Natl Acad Sci USA 100: 10483–10487.
Weinberger DR, Egan MF, Bertolino A, Callicott JH, Mattay VS, Lipska BK et al (2001). Prefrontal neurons and the genetics of schizophrenia. Biol Psychiatry 50: 825–844.
Wu DC, Jackson-Lewis V, Vila M, Tieu K, Teismann P, Vadseth C et al (2002). Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J Neurosci 22: 1763–1771.
Yee BK, Chang DL, Feldon J (2004). The Effects of dizocilpine and phencyclidine on prepulse inhibition of the acoustic startle reflex and on prepulse-elicited reactivity in C57BL6 mice. Neuropsychopharmacology 29: 1865–1877.
Yong VW, Wells J, Giuliani F, Casha S, Power C, Metz LM (2004). The promise of minocycline in neurology. Lancet Neurol 3: 744–751.
Yrjanheikki J, Keinanen R Pellikka M, Hokfelt T, Koistinaho J (1998). Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci USA 95: 15769–15774.
Yrjanheikki J, Tikka T, Keinanen R, Goldsteins G, Chan PH, Koistinaho J (1999). A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci USA 96: 13496–134500.
Zhang L, Kitaichi K, Fujimoto Y, Nakayama H, Shimizu E, Iyo M et al (2006a). Protective effects of minocycline on behavioral changes and neurotoxicity in mice after administration of methamphetamine. Prog Neuropharmacol Biol Psychiatry 30: 1381–1393.
Zhang L, Shirayama Y, Shimizu E, Iyo M, Hashimoto K (2006b). Protective effects of minocycline on 3,4-methylenedioxymethamphetamine-induced neurotoxicity in serotonergic and dopaminergic neurons of mouse brain. Eur J Pharmacol 544: 1–9.
Zhu S, Stavrovskaya IG, Drozda M, Kim BY, Ona V, Li M et al (2002). Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature 417: 74–78.
Acknowledgements
This study was supported in part by grants from the Minister of Education, Culture, Sports, Science, and Technology of Japan (KH), the Ministry of Health, Labor and Welfare of Japan (KH), and the Program for Promotion of Fundamental Studies in Health Sciences of the National Institutes of Biomedical Innovation (KH). We thank Dr Nori Takei (Department of Psychiatry, Hamamatsu University School of Medicine) for his valuable suggestion on statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, L., Shirayama, Y., Iyo, M. et al. Minocycline Attenuates Hyperlocomotion and Prepulse Inhibition Deficits in Mice after Administration of the NMDA Receptor Antagonist Dizocilpine. Neuropsychopharmacol 32, 2004–2010 (2007). https://doi.org/10.1038/sj.npp.1301313
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1301313
Keywords
This article is cited by
-
A CCR5 antagonist, maraviroc, alleviates neural circuit dysfunction and behavioral disorders induced by prenatal valproate exposure
Journal of Neuroinflammation (2022)
-
Acute stress-induced change in polysialic acid levels mediated by sialidase in mouse brain
Scientific Reports (2019)
-
Preventive effects of minocycline in a neurodevelopmental two-hit model with relevance to schizophrenia
Translational Psychiatry (2016)
-
Minocycline exacerbates apoptotic neurodegeneration induced by the NMDA receptor antagonist MK-801 in the early postnatal mouse brain
European Archives of Psychiatry and Clinical Neuroscience (2016)
-
Tspyl2 Loss-of-Function Causes Neurodevelopmental Brain and Behavior Abnormalities in Mice
Behavior Genetics (2016)