Abstract
It is well established that hypothalamic–pituitary–adrenal (HPA) axis dysregulation, characterized by elevated circulating cortisol concentrations and impaired negative feedback inhibition, is associated with affective disorders. As normalization of the HPA axis function and mood-stabilizing effects occur simultaneously during antidepressant treatment, it is likely that these effects are either directly or indirectly dependent. Although data concerning the outward transport of glucocorticoids from the brain by P-glycoprotein (Pgp) are inconsistent, it has been hypothesized that antidepressants exert their clinical activity in parts by inhibiting Pgp, subsequently leading to enhanced brain glucocorticoid levels and the normalization of the HPA axis function. Here, we report on the effects of different antidepressants (amitriptyline, fluoxetine, mirtazapine, St John's wort extract) on the brain/plasma distribution of corticosterone in mice after acute and subchronic treatment. The four antidepressants exerted different effects on the corticosterone concentration in brain and plasma. Changes in corticosterone levels were highly correlated, suggesting passive diffusion between both tissues. St John's wort extract and fluoxetine elevated brain and plasma corticosterone concentrations after subchronic treatment. Mirtazapine and amitriptyline had no effect on corticosterone concentration after subchronic treatment, possibly because both are also potent antagonists at the 5-HT2 receptor, which mediates HPA axis stimulation by serotonergic stimuli. In addition, St John's wort is the only antidepressant tested which slightly elevated Pgp protein level in the brain.
Similar content being viewed by others
INTRODUCTION
Hypothalamic–pituitary–adrenal (HPA) axis dysregulation, characterized by elevated cortisol concentrations and changes in diurnal rhythms of cortisol secretion, are observed in many patients suffering from affective disorders (Holsboer, 2000; Gillespie and Nemeroff, 2005). Preclinical as well as clinical findings suggest that this may possibly be caused by an impaired feedback inhibition (Holsboer, 2000; Pariante, 2003).
Normalization of the HPA axis function and the mood-stabilizing effects seem to occur simultaneously during antidepressive treatment and after recovery, indicating that the two effects might be either directly or indirectly dependent. However, whether HPA axis dysfunction is cause or consequence of depression is still a matter of dispute. Nevertheless, the effects of long-term antidepressant treatment on HPA axis and feedback inhibition by glucocorticoids may be important in understanding the mechanism by which antidepressants exert their clinical activity. It has been hypothesized that antidepressants inhibit membrane glucocorticoid transporters, such as P-glycoprotein (Pgp), at the blood-brain barrier (BBB) (Schinkel et al, 1996) and hence enhance the glucocorticoid concentration in the brain, leading to an increased glucocorticoid receptor-mediated gene transcription and therefore to normalization of the function of the HPA axis (Pariante et al, 2001; Pariante et al, 2004b).
Pgp, a 170–180 kDa plasma membrane-associated protein, is a member of the superfamily of ATP-binding-cassette transporters (ABC transporters). At the BBB, Pgp is localized in the apical membrane of brain capillary endothelial cells and transports substrates towards the blood compartment (Schinkel et al, 1996). Therefore, Pgp can limit the penetration into and retention within the central nervous system (CNS) of numerous compounds, thus modulating their effectiveness and CNS toxicity (Weiss et al, 2003).
Data concerning the transport of glucocorticoids by Pgp, however, are controversial. Ueda et al (1992) found that cortisol, aldosterone and dexamethasone but not progesterone, are physiological substrates for Pgp. Another group reported that Pgp hampers the access of cortisol but not of corticosterone to mouse and human brain (Karssen et al, 2001). van Kalken et al (1993) demonstrated that Pgp may function as a transporter for cortisol. Uhr et al analyzed the transport of different glucocorticoids in mdr1a/b (−/−) mice, lacking Pgp. In these mice, corticosterone and cortisol penetration into the brain is limited by the presence of Pgp at the BBB (Uhr et al, 2002). If antidepressants induce the expression of Pgp or inhibit its function, they could therefore alter the distribution of glucocorticoids between brain and plasma.
MATERIALS AND METHODS
Materials
Extract of St John's wort LI 160 (containing 0.18% hypericin; 3.77% hyperforin and 5.92% flavonoids) was a kind gift of Lichtwer Pharma AG (Berlin, Germany). Fluoxetine and mirtazapine were kindly provided by Stada Arzneimittel AG (Bad Vilbel, Germany). The Correlate-EIA™ corticosterone enzyme immunoassay kit (Assay Designs Inc.) was purchased from Biotrend (Cologne, Germany). Anti-Pgp C219 was obtained from Alexis Biochemicals (Lausen, Switzerland). All other chemicals, culture media and substances were purchased in the highest grade commercially available.
Animal Study
In total, 50 male NMRI mice with body weights ranging between 25 and 35 g were supplied by Harlan–Winkelmann GmbH (Borchen, Germany). Animals were housed under standard conditions in cages (five mice per cage) and given standard chow diet and water ad libitum. Mice were equally devided into a control group (vehicle) and four verum groups receiving either St John's wort extract (LI160, Lichtwer), amitriptyline (Sigma), fluoxetine (Stada) or mirtazapine (Stada). All antidepressants were suspended in aqueous agarose gel (0.2%) for treatment. For the acute study, the animals were treated once and dissected 1 h after treatment. For the subchronic study, treatment was given daily for 2 weeks and the animals were killed on day 15 after a washout period.
The following doses were used for the acute study: St John's wort: 500 mg/kg; amitriptyline, fluoxetine and mirtazapine 10 mg/kg. For the subchronic study, the following doses were administered: St John's wort: 300 mg/kg; amitriptyline, fluoxetine and mirtazapine 10 mg/kg. Control mice received the weight-adjusted volume of vehicle. Treatment was given once daily by oral gavage via a pharyngeal tube (diameter 1 mm) with maximal application volume of 0.5 ml. Oral application was chosen as it is the standard application of antidepressants and St John's wort extract. Mice were weighed daily for dose adjustment.
All experiments were carried out according to the guidelines of the German Protection of Animals Act (Deutsches Tierschutzgesetz, BGBI 1998, Part I, No. 30, S. 1105 ff) by individuals with appropriate training and experience.
Blood samples were collected retrobulbarly in tubes containing 0.03 ml heparin to avoid coagulation and subsequently centrifuged to obtain the serum fraction. Mice were then sacrificed by decapitation. The plasma and weighed cerebrum samples were collected and stored at −80°C until analysis.
Sample Preparation
Brains were thawed and subsequently homogenized (1000 r.p.m., × 10) using the buffer supplied with the corticosterone EIA kit (300 mg wet weight (WW)=2 ml buffer). The homogenates were divided into two aliquots and centrifuged.
Western Blots
From one aliquot the supernatant was removed. The pellet was resuspended in STEN buffer and recentrifuged after vortexing. The protein concentration in the supernatants was determined according to Lowry et al (1951).
Corticosterone EIA
Corticosteroid displacement reagent was added to the supernatants of the second brain homogenate aliquot and was subsequently diluted with the respective assay buffer. For blood corticosterone analysis, 100 μl of the plasma were mixed with steroid displacement reagent and afterwards diluted with the respective assay buffer.
Western Blots
A previously published protocol was used for Western blot analysis (Keil et al, 2004). Briefly, after dilution in sample buffer, 10–20 μg of protein were loaded on an acrylamide gel (Invitrogen, Karlsruhe, Germany) and examined by SDS-PAGE. The proteins were transferred on PVDF-membranes (Amersham Bioscience, Uppsala, Sweden) at 25 V for 90 min. Thereafter, membranes were saturated with 5% nonfat dry milk in TBST for 1 h. PVDF membranes were then exposed to the primary antibody (C219) over night. After treatment for 1 h with the corresponding secondary antibody (Merck Bioscience, Darmstadt, Germany), protein bands were detected by ECL reagent (Amersham Bioscience, Uppsala, Sweden).
Blots were scanned and the band intensity was determined after background subtraction using Scion Image for windows beta 4.0.2 (Frederick, USA).
Corticosterone EIA
The Correlate-EIA™ Corticosterone kit is a competitive immunoassay for the quantitative determination of corticosterone in biological fluids. The kit uses a polyclonal corticosterone antibody to bind corticosterone in the standard or sample or an alkaline phosphatase molecule with covalently attached corticosterone. The sensitivity of the assay is 26.99 pg/ml (http://www.assaydesigns.com/products/catalog/inserts/900-097.pdf). The brain and plasma levels seen in NMRI (this paper) are within the ranges seen previously for several other mouse strains (Butte et al, 1972).
Samples and standards were treated according to the manufacturer's instructions. After adding the stop solution, the plate was immediately placed in a micro plate reader (1420 Wallac Victor2, Perkin-Elmer, Rodgau-Jügesheim, Germany). Absorbance was read at 405 nm. Each sample and standard was measured in duplicates.
The following control values were determined: Blank, nonspecific binding and total activity. A standard curve was determined on every plate.
Statistics
Data are given as mean±SEM. Statistical analysis was performed using t-test against the untreated control. p-value ⩽0.05 was considered significant. Linear regressions and correlations were calculated using GraphPad Prism 4.02 (San Diego, USA).
RESULTS
As the data concerning transport of glucocorticoids by Pgp are conflicting, we previously analyzed the inhibition of calcein-AM uptake of cortisol and corticosterone in CEM/VLB cells (human lymphocytic leukemia cell lines) and PBCEC cells (porcine brain capillary endothelial cells). Both cell lines were used to characterize the transport of several antidepressant drugs by Pgp (Weber et al, 2005; Weber et al, 2004). Cortisol is a weak and corticosterone a slightly more potent inhibitor of the transport function of Pgp (cortisol 100 μM: 14.7±3.1% (VLB); 21.0±8.4% (PBCEC); corticosterone 100 μM: 54.3±6.4% (VLB); 82.2±9.5% (PBCEC) inhibition of calcein-AM uptake) (data not shown).
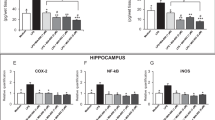
As changes in the expression of Pgp at the BBB could influence corticosterone brain levels, we additionally investigated the effects of all treatment conditions on Pgp expression. After acute treatment, we found no significant differences between the individual groups (data not shown). Only St John's wort extract significantly increased the expression of Pgp after subchronic application. The other antidepressants, that is amitriptyline, fluoxetine and mirtazapine, had no effect (Figure 1). An in vitro model confirmed the increase of Pgp expression by St John's wort extract. Both St John's wort extract and hyperforin increased the protein levels of Pgp in the CEM cells after 24 h incubation (St John's wort extract 10 μg/ml: +65.5%; St John's wort extract 25 μg/ml: +84.0%; hyperforin 0.1 μM: +26.3%; hyperforin 1 μM: +52.2%) (data not shown).
All animals, control groups and the four treatment groups, gained weight (about 3–4 g), during the subchronic treatment study. However, no statistically significant differences were observed between groups.
The possible effect of treatment-associated stress on the plasma or brain corticosterone levels were assessed by comparing animals treated orally under control conditions (0.2% agarose only) with animals not treated at all. There were no significant differences detectable between these groups (Figure 2).
Fluoxetine, mirtazapine and St John's wort extract, but not amitriptyline, significantly elevated plasma and brain corticosterone levels after one single oral treatment (Figures 3, 4, 5 and 6). However, in subchronic treatment, only fluoxetine and St John's wort extract significantly elevated plasma as well as brain corticosterone levels. Mirtazapine and amitriptyline showed no effect (Figure 3 4, 5 and 6).
Interestingly, over all subchronic treatment conditions, changes of plasma corticosterone always led to parallel changes of the respective brain concentrations (Figure 7). Brain concentration (ng/g WW) was always about 70% of the plasma levels (ng/ml) suggesting equal distribution in the extra—as well as the intracellular water space. After acute treatment, the brain concentrations after 1 h were slightly lower than, but linearly correlated to, plasma concentrations (Figure 7).
Correlation of brain and plasma corticosterone levels in the four treatment (St John's wort, amitriptyline, fluoxetine and mirtazapine) and control groups after acute (upper panel) and subchronic (lower panel) treatment. Linear regression was performed using GraphPad Prism (acute: slope: 0.396±0.05; r2: 0.5619 and subchronic: slope: 0.278±0.05; r2: 0.3708).
DISCUSSION
The present study shows that the brain corticosterone concentration in the mouse is a linear function of its plasma concentration and that brain levels (in ng/g WW) are usually slightly less than the plasma levels (in ng/ml). These support the assumption that corticosterone diffuses passively into the brain, mainly into the intra- and extracellular water space. Any contribution of an outward transport by Pgp, if present, can only be of minor importance. Thus, it appears rather unlikely that inhibition of Pgp by some of the antidepressants used has any relevant effect on brain levels of corticosterone in the mouse. The differences in the brain and plasma concentrations after acute treatment may be explained by the time lag it takes for diffusion into the brain.
Our findings that antidepressants have different acute or chronic effects on plasma levels of glucocorticoids confirm and extent several other findings in humans or lab animals.
A previous study on the effect of fluoxetine on plasma corticosterone concentration in rats yielded inconsistent results (Stout et al, 2002). The data of our study, however, clearly indicate that the administration of fluoxetine enhances brain and plasma corticosterone levels after acute and subchronic treatment. These results coincide with those previously shown in resting and acutely stressed rats (Berton et al, 1999), female Mongolian gerbils (Hendrie et al, 2003), and humans (Von Bardeleben et al, 1989).
Furthermore, neither acute nor subchronic administration of amitriptyline influenced brain or plasma corticosterone levels. These results agree with a previous human study which showed that amitriptyline did not influence the plasma cortisol concentration in patients suffering from DSM-IV major depressive disorder (Rota et al, 2005). Reul et al (1993) in contrast, found that long-term oral treatment with amitriptyline significantly decreased basal as well as stress-induced corticosterone plasma level in rats.
Acute administration of mirtazapine led to an elevation of corticosterone in brain and plasma. However, after 14 days of treatment, no differences of corticosterone plasma levels were observed, in comparison to control animals. Previously, mirtazapine (15 ms) has been shown to decrease plasma cortisol after acute administration in healthy male subjects (Laakmann et al, 1999). In another study with depressed patients, mirtazapine seemed to reduce plasma cortisol after 5 weeks of treatment (Schule et al, 2003).
Similar to fluoxetine, St John's wort extract increased plasma as well as brain corticosterone levels after acute and subchronic treatment. These data agree with most previous findings in animals and humans (Franklin, 2005). Franklin et al (2004), for example, treated rats with about 300 mg/kg St John's wort extract via food pellets for two weeks. Whereas plasma corticosterone was not elevated, brain corticosterone was reduced by about 30%. The authors suggested that upregulation of Pgp by St John's wort extract at the BBB might explain their findings. In our experiments, however, oral treatment led to a substantial elevation of plasma as well as brain corticosterone. Although small Pgp upregulation in the St John's wort-treated animals were observed, they seem too small, however, to explain these results. Brain corticosterone regulation might therefore differ between mice and rat, also indicated by 25–30 times higher brain vs plasma levels of corticosterone in the rat (Franklin et al, 2004) compared to the nearly one-to-one relationship in the mouse (this work).
Antidepressants, such as reboxetine, increase cortisol secretion by enhancing serotoninergic and noradrenergic neurotransmission (Schule et al, 2004). Serotoninergic stimulation seems to be mediated mainly via 5-HT2A receptors (Feldman et al, 1998; Bagdy, 1996). This may explain why amitriptyline and mirtazapine do not enhance plasma corticosterone, since both are also potent 5-HT2A antagonists (Sanchez and Hyttel, 1999).
In summary, fluoxetine, mirtazapine, amitryptiline and St John's wort exerted in the two test regimes different effects on brain corticosterone levels. Our observations do not support the hypothesis presented by Pariante et al (2004a, 2004b) and Pariante et al (2001) that normalization of the disturbed feedback inhibition of the HPA axis by enhancing cerebral glucocorticoid concentration represents a rather common mechanism of action of most antidepressant drugs.
References
Bagdy G (1996). Role of the hypothalamic paraventricular nucleus in 5-HT1A, 5-HT2A and 5-HT2C receptor-mediated oxytocin, prolactin and ACTH/corticosterone responses. Behav Brain Res 73: 277–280.
Berton O, Durand M, Aguerre S, Mormede P, Chaouloff F (1999). Behavioral, neuroendocrine and serotonergic consequences of single social defeat and repeated fluoxetine pretreatment in the Lewis rat strain. Neuroscience 92: 327–341.
Butte JC, Kakihana R, Noble EP (1972). Rat and mouse brain corticosterone. Endocrinology 90: 1091–1100.
Feldman S, Newman ME, Gur E, Weidenfeld J (1998). Role of serotonin in the amygdala in hypothalamo–pituitary–adrenocortical responses. Neuroreport 9: 2007–2009.
Franklin M (2005). Endocrinology of St John's wort. In: Muller WE (ed). St John's Wort and its Active Principles in Depression and Anxiety. Birkhäuser Verlag: Basel. pp 161–181.
Franklin M, Reed A, Murck H (2004). Sub-chronic treatment with an extract of hypericum perforatum (St John's wort) significantly reduces cortisol and corticosterone in the rat brain. Eur Neuropsychopharmacol 14: 7–10.
Gillespie CF, Nemeroff CB (2005). Hypercortisolemia and depression. Psychosom Med 67(Suppl 1): S26–S28.
Hendrie CA, Pickles AR, Duxon MS, Riley G, Hagan JJ (2003). Effects of fluoxetine on social behaviour and plasma corticosteroid levels in female Mongolian gerbils. Behav Pharmacol 14: 545–550.
Holsboer F (2000). The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology 23: 477–501.
Karssen AM, Meijer OC, van dS I, Lucassen PJ, de Lange EC, de Boer AG et al (2001). Multidrug resistance P-glycoprotein hampers the access of cortisol but not of corticosterone to mouse and human brain. Endocrinology 142: 2686–2694.
Keil U, Bonert A, Marques CA, Scherping I, Weyermann J, Strosznajder JB et al (2004). Amyloid β-induced changes in nitric oxide production and mitochondrial activity lead to apoptosis. J Biol Chem 279: 50310–50320.
Laakmann G, Schule C, Baghai T, Waldvogel E (1999). Effects of mirtazapine on growth hormone, prolactin, and cortisol secretion in healthy male subjects. Psychoneuroendocrinology 24: 769–784.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951). Protein measurement with the folin phenol reagent. J Biol Chem 193: 265–275.
Pariante CM (2003). Depression, stress and the adrenal axis. J Neuroendocrinol 15: 811–812.
Pariante CM, Makoff A, Lovestone S, Feroli S, Heyden A, Miller AH et al (2001). Antidepressants enhance glucocorticoid receptor function in vitro by modulating the membrane steroid transporters. Br J Pharmacol 134: 1335–1343.
Pariante CM, Papadopoulos AS, Poon L, Cleare AJ, Checkley SA, English J et al (2004a). Four days of citalopram increase suppression of cortisol secretion by prednisolone in healthy volunteers. Psychopharmacology (Berlin) 177: 200–206.
Pariante CM, Thomas SA, Lovestone S, Makoff A, Kerwin RW (2004b). Do antidepressants regulate how cortisol affects the brain? Psychoneuroendocrinology 29: 423–447.
Reul JM, Stec I, Soder M, Holsboer F (1993). Chronic treatment of rats with the antidepressant amitriptyline attenuates the activity of the hypothalamic–pituitary–adrenocortical system. Endocrinology 133: 312–320.
Rota E, Broda R, Cangemi L, Migliaretti G, Paccotti P, Rosso C et al (2005). Neuroendocrine (HPA axis) and clinical correlates during fluvoxamine and amitriptyline treatment. Psychiatry Res 133: 281–284.
Sanchez C, Hyttel J (1999). Comparison of the effects of antidepressants and their metabolites on reuptake of biogenic amines and on receptor binding. Cell Mol Neurobiol 19: 467–489.
Schinkel AH, Wagenaar E, Mol CA, van Deemter L (1996). P-glycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J Clin Invest 97: 2517–2524.
Schule C, Baghai T, Schmidbauer S, Bidlingmaier M, Strasburger CJ, Laakmann G (2004). Reboxetine acutely stimulates cortisol, ACTH, growth hormone and prolactin secretion in healthy male subjects. Psychoneuroendocrinology 29: 185–200.
Schule C, Baghai T, Zwanzger P, Ella R, Eser D, Padberg F et al (2003). Attenuation of hypothalamic–pituitary–adrenocortical hyperactivity in depressed patients by mirtazapine. Psychopharmacology (Berlin) 166: 271–275.
Stout SC, Owens MJ, Nemeroff CB (2002). Regulation of corticotropin-releasing factor neuronal systems and hypothalamic–pituitary–adrenal axis activity by stress and chronic antidepressant treatment. J Pharmacol Exp Ther 300: 1085–1092.
Ueda K, Okamura N, Hirai M, Tanigawara Y, Saeki T, Kioka N et al (1992). Human P-glycoprotein transports cortisol, aldosterone, and dexamethasone, but not progesterone. J Biol Chem 267: 24248–24252.
Uhr M, Holsboer F, Muller MB (2002). Penetration of endogenous steroid hormones corticosterone, cortisol, aldosterone and progesterone into the brain is enhanced in mice deficient for both mdr1a and mdr1b P-glycoproteins. J Neuroendocrinol 14: 753–759.
van Kalken CK, Broxterman HJ, Pinedo HM, Feller N, Dekker H, Lankelma J et al (1993). Cortisol is transported by the multidrug resistance gene product P-glycoprotein. Br J Cancer 67: 284–289.
von Bardeleben U, Steiger A, Gerken A, Holsboer F (1989). Effects of fluoxetine upon pharmacoendocrine and sleep-EEG parameters in normal controls. Int Clin Psychopharmacol 4(Suppl 1): 1–5.
Weber CC, Kressmann S, Fricker G, Muller WE (2004). Modulation of P-glycoprotein function by St John's wort extract and its major constituents. Pharmacopsychiatry 37: 292–298.
Weber CC, Kressmann S, Ott M, Fricker G, Müller WE (2005). Inhibition of P-glycoprotein function by several antidepressants may not contribute to clinical efficacy. Pharmacopsychiatry 38: 293–300.
Weiss J, Dormann SM, Martin-Facklam M, Kerpen CJ, Ketabi-Kiyanvash N, Haefeli WE (2003). Inhibition of P-glycoprotein by newer antidepressants. J Pharmacol Exp Ther 305: 197–204.
Acknowledgements
This study was supported by Lichtwer Pharma AG (Berlin, Germany).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Weber, CC., Eckert, G. & Müller, W. Effects of Antidepressants on the Brain/Plasma Distribution of Corticosterone. Neuropsychopharmacol 31, 2443–2448 (2006). https://doi.org/10.1038/sj.npp.1301076
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1301076
Keywords
This article is cited by
-
Fluoxetine effects on molecular, cellular and behavioral endophenotypes of depression are driven by the living environment
Molecular Psychiatry (2017)
-
Involvement of Pituitary Adenylate Cyclase Activating Polypeptide (PACAP) and its Receptors in the Mechanism of Antidepressant Action
Journal of Molecular Neuroscience (2008)
-
The Antidepressant Desipramine Requires the ABCB1 (Mdr1)-Type p-Glycoprotein to Upregulate the Glucocorticoid Receptor in Mice
Neuropsychopharmacology (2007)