Abstract
Dopamine plays a role in the pathophysiology of depression and therapeutic effects of antidepressants but the contribution of individual D2-like receptor subtypes (D2, D3, D4) to depression is not known. We present evidence that activation of D2/D3, but not D4 receptors, can affect the outcome in the rat forced swim test (FST). Nomifensine, a dopamine uptake inhibitor (7, 14, and 28 μmol/kg); quinpirole, a D2-like receptor and agonist (0.4, 1.0, and 2.0 μmol/kg); PD 12,8907, a preferential D3 receptor agonist (0.17, 0.35, and 0.7 μmol/kg); PD 168077 (0.1, 0.3, and 1.0 μmol/kg) and CP 226269 (0.3, 1.0, and 3.0 μmol/kg), both selective D4 receptor agonists, were administered s.c. 24, 5, and 0.5/1 h before testing. Nomifensine, quinpirole at all doses and PD 128907 at the highest dose decreased immobility time in FST. PD 168077 and CP 226269 had no effect on the model. To further clarify what type of dopamine receptors were involved in the anti-immobility effect of quinpirole, we tested different antagonists. Haloperidol, a D2-like receptor antagonist (0.27 μmol/kg), completely blocked the effect of quinpirole; A-437203 (LU-201640), a selective D3 receptor antagonist (17.46 μmol/kg), showed a nonsignificant trend to attenuate the effect of the low dose of quinpirole, and L-745,870, a selective D4 receptor antagonist (1.15 μmol/kg), had no effect. The pharmacological selectivity of the compounds tested suggests that the antidepressant-like effects of quinpirole are most likely mediated mainly by D2 and to a lesser extent by D3 but not D4 receptors.
Similar content being viewed by others
INTRODUCTION
Depression has been associated with the dysfunction of neurotransmitter systems, mainly norepinephrine and serotonin. Dopamine is also proposed to play an important role in the pathophysiology of depression as well as in the mechanism of action of antidepressant drugs (Willner, 1983a, 1983b; Borsini et al, 1985a, 1985b; Pulvirenti and Samanin, 1986; Fibiger, 1995; Charney, 1998; Naranjo et al, 2001; Klimek et al, 2002; Pania and Gessab, 2002). Mesolimbic dopamine pathways are involved in the control of motivation and reward-related behaviors (Koob, 1996; Robbins and Everitt, 1996; Schultz, 1997; Berridge and Robinson, 1998) and hypofunction of the dopamine system is implicated in the loss of motivation and/or anhedonia described as core symptoms in human depressive states (Nelson and Charney, 1981). Administration of dopamine receptor antagonists, or drugs that reduce dopamine levels such as reserpine, induces dysphoria and many symptoms resembling those of endogenous depression (Wise et al, 1978; Willner, 1983a). Conversely, dopamine receptor agonists, as well as drugs that increase dopamine function, have antidepressant-like profiles in animal models of depression (Muscat et al, 1992) and have been reported to have efficacy in the treatment of human depression (Forrest et al, 1977; Bouras and Bridges, 1982; Lopez-Ibor Alino et al, 1982; Kinney, 1985). Moreover, long-term administration of antidepressant drugs or repeated administration of electroconvulsive shock (ECS) increases behavioral response to dopaminergic agonists and dopaminergic neurotransmission in the limbic system (Spyraki and Fibiger, 1981; Willner and Montgomery, 1981; Maj et al, 1984a, 1984b, 1998b; Serra et al, 1992; Ainsworth et al, 1998a).
Dopamine receptors can be classified into two families: the D1-like receptors (D1 and D5) and the D2-like receptors (D2, D3, and D4 receptor subtypes). Previous studies have reported the role of dopamine in the mechanism of action of antidepressants, focusing on behavioral responses to dopamine agonists after chronic antidepressant treatment or on how selective dopamine receptor antagonists can affect the ability of antidepressants to elicit their behavioral response (Willner and Montgomery, 1981; Maj et al, 1984b, 1989; Serra et al, 1990; Gambarana et al, 1995; D'Aquila et al, 2000a). For example, dopamine agonists have shown efficacy in models of behavioral despair and chronic mild stress (Porsolt et al, 1979; Duterte-Boucher et al, 1988; Muscat et al, 1992). Most of the studies have assigned a critical role to D2-like receptors, as compared to D1-like receptors (Spyraki and Fibiger, 1981; Borsini et al, 1988; Maj et al, 1989, 1996a; Nunes Junior et al, 1994; Ainsworth et al, 1998b; Rogoz and Dziedzicka-Wasylewska, 1999); however, little is known about the involvement of the individual dopamine receptor subtypes within the D2-like family in preclinical models of depression such as the forced swim test (FST). The involvement of the D3 receptor in antidepressant activity has been proposed previously based on the preclinical and clinical effects of compounds that activate the D3 receptor as well as a change in the D3 receptor functioning following chronic antidepressant treatments (Sokoloff et al, 1992; Levant et al, 1993; Willner et al, 1994; Maj et al, 1997, 1998a; Corrigan et al, 2000). Interestingly, although the D4 receptor has been proposed as a potential target for schizophrenia treatment, its relevance in other psychiatric disorders such as depression has yet to be investigated.
The rat FST is one of the most widely used animal models for assessing antidepressant-like activity (Porsolt et al, 1978; Porsolt et al, 1979). This model is based on the principle that an animal exposed to an inescapable stressor will show altered behavior in response to subsequent stressors. Rats are placed in cylinders filled with water. After initial vigorous attempts to struggle and escape, they develop an immobile posture, which has been called behavioral despair and has been hypothesized to reflect lowered mood or hopelessness. Upon subsequent exposure, rats become immobile faster and for a longer period of time than naive animals, suggestive of a ‘depressive-like state’. FST is sensitive to a broad variety of antidepressant treatments, and they significantly reduce the immobility period (Porsolt et al, 1979). Furthermore, Detke et al (1995) has proposed that the rats can display various behavioral patterns in this model, differentially related to different classes of antidepressant drugs.
The goal of this study was to investigate the involvement of each of the D2-like receptors subtypes (D2, D3, and D4) in an animal model of depression considered to have good predictive value for detecting antidepressant activity, FST, using selective dopamine receptor agonists and/or antagonists. We compared the effects of nomifensine, a dopamine uptake inhibitor (Meiergerd and Schenk, 1994); quinpirole, a D2-like receptor agonist (Ki=38.4, 4.5, and 91.0 nM for D2, D3, and D4 receptors, respectively) (Moreland et al, 2004); PD 128907, a preferential D3 receptor agonist (Ki=15.1, 1.1, and 1040.0 nM for D2, D3, and D4 receptors, respectively) (Moreland et al, 2004); PD 168077 (Ki=1050.0, 2540.0, and 11.9 nM for D2, D3, and D4 receptors, respectively) and CP 226269 (Ki=120.0, 521.0, and 0.8 nM for D2, D3, and D4 receptors, respectively), both selective D4 receptor agonists (Moreland et al, 2004). To further clarify the role of specific dopamine receptors in the anti-immobility effect of quinpirole, we assessed the ability of haloperidol, a nonselective D2-like receptor antagonist (Ki=0.3, 3.6 and 1.9 nM for D2, D3, and D4 receptors, respectively) (Moreland et al, 2004); A-437203 (LU-201640), a D3 receptor antagonist (Ki=71.0, 1.6, and 6220.0 nM for D2, D3, and D4 receptors, respectively) (Unger et al, 2002; Chaperon et al, 2003); and L-745,870, a highly selective D4 receptor antagonist (Ki=>10 000, >10 000 and 0.4 nM for D2, D3, and D4 receptors, respectively) (Moreland et al, 2004; Patel et al, 1997) to attenuate the quinpirole-induced antidepressant-like response. Results support a major role for dopamine D2 receptors, a moderate role for D3 receptors, and no involvement of D4 dopamine receptors in antidepressant-like effects in the rat FST.
MATERIALS AND METHODS
Animals
Male Sprague–Dawley rats (Charles River Laboratories, Inc.) weighing 250–350 g were used for these experiments. Animals were allowed to habituate to our facilities at least 1 week prior the start of the experiments. Rats were group housed (five per cage) and maintained on a 12 : 12-h light–dark schedule (lights on 0600, lights off 1800), in a temperature- and humidity-controlled environment (22±1°C, 60–70% humidity). Animals had free access to food and water. All studies were approved by the Abbott Laboratories Institutional Animal Care and Use Committee, according to the guidelines of the Association for the Assessment and Accreditation of Laboratory Animals Care.
Drugs
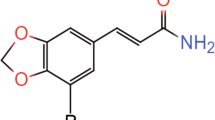
(−)-Quinpirole hydrochloride (Sigma, St Louis, MO), PD 168077, and CP 226269 (both synthesized at Abbott Laboratories) were dissolved in 0.1% ascorbic acid in saline solution (0.9%). Nomifensine maleate salt (Sigma, St Louis, MO) was dissolved in 10% DMSO and 90% hydroxypropyl β-cyclodextrin. Haloperidol (Sigma, St Louis, MO) was dissolved in acetic acid and water, and pH was adjusted to 5.0–5.5 with NaOH. A-437203 (LU-201640), a D3 receptor antagonist synthesized at Abbott Laboratories, was dissolved in NaOH and sterile water and pH was adjusted to 10.5 with 1 N HCl. Imipramine hydrochloride (Sigma, St Louis, MO), L-745,870 trihydrochloride, and PD 128907 (both from Tocris, Ellisville, MO) were dissolved in sterile water.
Forced Swim Test
The procedure was based on the behavioral test described by Porsolt et al (1978). A single experiment consisted of a preswim and a test swim. Naive rats were individually placed inside vertical cylinders (height: 40 cm, diameter: 30 cm) containing 25 cm of water at 23–25°C for 15 min. Following this preswim, animals were removed and allowed to dry in a heated enclosure before returning to their home cages. After 24 h, the test swim occurred in which the rats were replaced in the cylinder for 5 min and the total duration of immobility and escape behaviors was measured. Test swims were videotaped and subsequently assessed for the following behaviors: immobility (the animal remains floating passively in the water without struggling and shows the minimal movements necessary to keep its head above water), climbing (very vigorous, active movements with animal's forepaws breaking the water surface usually against the walls of the water container), and swimming (described as active movements more than those necessary to keep the head of the rat above the water, and mainly distinguished as propelling the rat around the cylinder). Total time spent engaged in each activity was recorded. Both swimming sessions were performed during the afternoon between 1200 and 1600 h. Animals were used only once in a single experiment including preswim and test swim.
Locomotor Activity
Animals were habituated to the testing room under dim lighting conditions for 1.5 h before drug administration and testing. Locomotor activity was measured by individually placing naive rats into automated activity chambers (Versamax from Accuscan, Columbus, OH), measuring 42 cm × 30 cm × 42 cm (length × height × depth) immediately following drug application. Activity was detected by infrared photo beam sensors placed at 4.5 cm and 15.5 cm above the cage floor. Data were expressed as total distance moved during the 90-min testing period. Locomotor activity experiments were performed between 1000 and 1600 h.
Experimental Procedure
Forced swim test
For most of the experiments, a reference compound, imipramine 95 μmol/kg (30 mg/kg) was tested. Imipramine was administered i.p. 24, 5, and 1 h before the test swim. In all the experiments, the first administration of the compound/s took place 15 min after the 15-min preswim test. Nomifensine (7, 14, and 28 μmol/kg=2.5, 5, and 10 mg/kg) was administered i.p. 24, 5, and 1 h before the test swim. Quinpirole (0.4, 1.0, and 2.0 μmol/kg=0.1, 0.25, and 0.5 mg/kg), PD 128907 (0.17, 0.35, and 0.7 μmol/kg=0.05, 0.1, and 0.2 mg/kg), PD 168077 (0.1, 0.3, and 1.0 μmol/kg=0.045, 0.135, and 0.45 mg/kg), and CP 226269 (0.3, 1.0, and 3.0 μmol/kg=0.13, 0.43, and 1.28 mg/kg) were administered s.c. 24, 5, and 0.5 h before the test swim. Haloperidol (0.27, 1.33, and 2.66 μmol/kg=0.1, 0.5, and 1.0 mg/kg i.p.), A-437203 (LU-201640) (0.52, 1.75, 5.24, and 17.46 μmol/kg=0.3, 1.0, 3.0, and 10.0 mg/kg i.p.), and L-745,870 (0.23, 1.15, 2.3, and 5.7 μmol/kg=0.1, 0.5, 1.0, and 2.5 mg/kg i.p.) were tested initially alone in order to determine effective dose ranges. In those experiments, haloperidol, A-437203, and L-745,870 were administered i.p. 24, 5, and 0.5 h before the test swim. In the subsequent antagonism experiments, haloperidol (0.27 μmol/kg), A-437203 (17.46 μmol/kg) or L-745,870 (1.15 μmol/kg) were injected i.p. 15 min prior to each quinpirole injection (0.4 and 1.0 μmol/kg s.c.).
Locomotor activity
To differentiate the anti-immobility effect of quinpirole from general locomotor activation, locomotor activity was evaluated in rats treated acutely with quinpirole 0.4, 1.0, and 2.0 μmol/kg, as well as following a subchronic quinpirole treatment according to the same protocol treatment used for rat FST (three injections, 0.4 and 1.0 μmol/kg).
Statistical Analyses
Experimental design for FST experiments were between-subjects, where each animal was observed for three different behaviors: immobility, climbing, and swimming. The number of rats in each experimental group was n=10, except for quinpirole (n=8–10), PD 168077 (n=8–9), haloperidol (n=9), and the combined treatment haloperidol+quinpirole experiment (n=15). Data for the FST experiments testing dopamine agonists or the dopamine uptake inhibitor were analyzed using a one-way ANOVA for treatment groups including in the analysis the data for the reference compound imipramine. When the ANOVA revealed a significant effect of treatment, post hoc analysis for individual group comparisons were followed (Fisher's protected least significant difference test). Those experiments using an antagonist to block the effect of quinpirole were analyzed using a two-way ANOVA where the main factors under consideration were antagonist (vehicle or antagonist) and quinpirole (different doses tested). When a statistically significant antagonist × quinpirole interaction was revealed, individual group comparisons followed the overall ANOVA (Fisher's protected least significant difference test). When no significant antagonist × quinpirole interaction was achieved but significant main effects were observed, the main effect of the independent variables were analyzed using a post hoc Fisher's protected least significant difference test, for the collapsed data over the levels of all other factors in the design.
For the locomotor activity study, data for distance moved were analyzed using a one-way ANOVA for treatment, followed by a post hoc analysis (Fisher's protected least significant difference test).
Data are expressed as the mean±SEM. For all tests, significance was defined as p<0.05.
RESULTS
Effect of Nomifensine, a Selective Dopamine Uptake Inhibitor, on Rat FST
ANOVA revealed a significant treatment effect for immobility time (F4, 45=35.403, p<0.001), climbing time (F4, 45=28.735, p<0.001), and swimming time (F4, 45=9.711, p<0.001). Post hoc mean contrast indicated a significant decrease in immobility time and a significant increase in climbing behavior for the three doses of nomifensine and for the reference compound imipramine compared to vehicle control group (p<0.001; Figure 1). Post hoc analysis for swimming time showed that nomifensine 7 and 14 μmol/kg increased swimming time (p<0.05 and p<0.001, respectively), while the higher dose of nomifensine (28 μmol/kg) interestingly did not (Figure 1). It is important to mention that nomifensine did not increase locomotor activity at the dose of 7 μmol/kg or lower. However, nomifensine at 14 and 28 μmol/kg induced a significant increase in locomotor activity (data not shown).
Effect of nomifensine, a selective dopamine uptake inhibitor on rat FST. Graph represents mean±SEM for immobility, climbing, and swimming time during the 5-min test swim. Nomifensine (7, 14, and 28 μmol/kg i.p.) was administered 24, 5, and 1 h before testing (n=10/group). *p<0.05 and ***p<0.001 compared to vehicle-treated rats (Fisher post hoc test). Imi=imipramine.
Effect of Quinpirole, a D2-Like Receptor Agonist, on Rat FST
A one-way ANOVA indicated a significant treatment effect for immobility time (F4, 43=24.986, p<0.001), and a significant treatment effect for climbing behavior (F4, 43=22.567, p<0.001; Figure 2). Post hoc mean comparisons revealed a significant decrease in immobility time at all doses of quinpirole and for the reference compound imipramine compared to the vehicle group (p<0.001), and a significant increase in climbing behavior compared to control rats (p<0.01 for quinpirole 0.4 μmol/kg and p<0.001 for quinpirole 1.0 and 2.0 μmol/kg and imipramine). ANOVA also indicated a significant treatment effect for swimming time (F4, 43=9.171, p<0.001), while mean comparisons showed a significant decrease in swimming time for quinpirole 1.0 μmol/kg (p<0.05), 2.0 μmol/kg (p<0.01), and for imipramine (p<0.05).
Effect of quinpirole, a D2-like agonist, on rat FST. Graph represents mean±SEM for immobility, climbing, and swimming time during the 5-min test swim. Quinpirole (0.4, 1.0, and 2.0 μmol/kg s.c.) was administered 24, 5, and 0.5 h before testing (n=8–10/group). *p<0.05, **p<0.01, and ***p<0.001 compared to vehicle-treated rats (Fisher post hoc test).
Effect of PD 128907, a Preferential D3 Receptor Agonist, on Rat FST
ANOVA revealed a significant treatment effect for immobility time (F4, 45=7.692, p<0.001), a significant treatment effect in climbing (F4, 45=8.928, p<0.001), but no significant effect in swimming (F4, 45=2.339, p=0.07; Figure 3a). Post hoc mean comparisons revealed a significant decrease in immobility time and a significant increase in climbing for the highest dose of PD 128907 (0.7 μmol/kg, p<0.001), as well as a significant effect of imipramine compared to vehicle group in both parameters (p<0.01 for immobility and p<0.001 for climbing). PD 128907 was tested in locomotor activity assay at the doses of 0.0425, 0.17, 0.7, 2.8, and 5.6 μmol/kg (0.0125, 0.05, 0.2, 0.8, and 1.6 mg/kg). All the doses of PD 128907 up to 2.8 μmol/kg (0.8 mg/kg) significantly reduced locomotor activity. PD 128907 5.6 μmol/kg (1.6 mg/kg) produced a nonsignificant trend towards increasing locomotor activity (data not shown), allowing separation between the antidepressant-like effects in FST from changes in spontaneous activity.
(a) Effect of PD 128907, a preferential D3 receptor agonist, on rat FST. PD 128907 (0.17, 0.35, and 0.7 μmol/kg s.c.) was administered 24, 5, and 0.5 h before testing (n=10/group). (b) Effect of PD 168077, a selective D4 agonist, on rat FST. PD 168077 (0.1, 0.3, and 1.0 μmol/kg s.c.) was administered 24, 5, and 0.5 h before testing (n=8–9/group). (c) Effect of CP 226269, a selective D4 agonist, on rat FST. CP 226269 (0.3, 1.0, and 3.0 μmol/kg s.c.) was administered 24, 5, and 0.5 h before testing (n=10/group). Graph represents mean±SEM for immobility, climbing, and swimming time during the 5-min test swim. *p<0.05, **p<0.01, and ***p<0.001 compared to vehicle-treated rats (Fisher post hoc test).
Effect of PD 168077 and CP 226269, Two Selective D4 Receptor Agonists, on Rat FST
A one-way ANOVA for PD 168077 data revealed a significant treatment effect for immobility time (F4, 37=3.829, p<0.05), a significant treatment effect in climbing (F4, 37=16.146, p<0.001), but no significant effect in swimming (Figure 3b). Post hoc mean comparisons revealed a significant decrease in immobility time and a significant increase in climbing only for the reference compound imipramine compared to vehicle group (p<0.001). PD 168077 failed to induce a significant effect in the FST at any of the doses tested.
A one-way ANOVA for CP 226269 data showed a significant treatment effect for immobility time (F4, 45=4.727, p<0.01), a significant treatment effect in climbing (F4, 45=19.543, p<0.001), and a significant effect in swimming (F4, 45=5.104, p<0.01, Figure 3c). Post hoc mean comparisons revealed only a significant decrease in immobility and swimming time (p<0.001 and p<0.05, respectively), and a significant increase in climbing (p<0.001) for the reference compound imipramine compared to vehicle group. CP 226269 did not elicit any significant change in the FST at any of the doses tested (Figure 3c).
Effect of Haloperidol, a D2-Like Receptor Antagonist, on Rat FST
No significant treatment effect was observed in any of the behaviors analyzed in the model at any of the tested doses of haloperidol (F3, 31=1.98, p=0.137 for immobility, F3, 31=1.059, p=0.381 for climbing, and F3, 31=0.969, p=0.42 for swimming; Table 1), although the highest dose of haloperidol (2.66 μmol/kg) showed a trend towards an increase in immobility time (Table 1). Considering that haloperidol can suppress spontaneous locomotor activity at doses higher than 0.27 μmol/kg (internal data), the dose of haloperidol 0.27 μmol/kg (0.1 mg/kg) was selected for subsequent studies.
Effect of Haloperidol on the Effect of Quinpirole on Rat FST
Figure 4 shows the effects of the pretreatment with a D2-like receptor antagonist, haloperidol (0.27 μmol/kg), on the effects induced by quinpirole in rat FST. Two-way ANOVA for immobility time indicated a significant antagonist treatment effect (F1, 84=129.19, p<0.001), a significant quinpirole effect (F2, 84=17.50, p<0.001), and a significant antagonist × quinpirole interaction (F2, 84=18.99, p<0.001). Post hoc analysis showed a significant reduction in immobility time of vehicle/quinpirole (0.4 and 1.0 μmol/kg) compared to vehicle/vehicle group (p<0.001). Haloperidol/vehicle-treated animals did not statistically differ from the control group (vehicle/vehicle) in immobility time; however, haloperidol pretreatment antagonized the effect on immobility induced by the two doses of quinpirole (p<0.001; Figure 4). Analysis of climbing behavior showed a significant antagonist treatment effect (F1, 84=105.64, p<0.001), a significant quinpirole effect (F2, 84=39.82, p<0.001), and a significant antagonist × quinpirole interaction (F2, 84=35.77, p<0.001). Post hoc analysis revealed that the vehicle/quinpirole group (0.4 and 1.0 μmol/kg) showed a significant increase in climbing behavior compared to the vehicle/vehicle group (p<0.01 and p<0.001, respectively) and these effects were blocked by pretreatment with haloperidol (p<0.001). The haloperidol/vehicle group (0.27 μmol/kg) did not show any statistically significant difference compared to the vehicle/vehicle control group in climbing behavior. Analysis of swimming behavior indicated a significant antagonist treatment effect (F1, 84=17.16, p<0.001), a significant quinpirole effect (F2, 84=10.48, p<0.001), and a significant antagonist × quinpirole interaction (F2, 84=9.68, p<0.001). Post hoc analysis revealed that the vehicle/quinpirole group (0.4 μmol/kg dose only) showed a significant increase in swimming behavior compared to the vehicle/vehicle group (p<0.001) and the effects observed at this lower dose were blocked by pretreatment with haloperidol (p<0.001).
Effect of haloperidol on the effect of quinpirole on rat FST. Graph represents mean±SEM for immobility, climbing, and swimming time during the 5-min test swim. Haloperidol (0.27 μmol/kg i.p.) was administered 15 min before each quinpirole injection. Quinpirole (0.4 and 1.0 μmol/kg s.c.) was injected 24, 5, and 0.5 h before testing (n=15/group). **p<0.01 and ***p<0.001 compared to vehicle/vehicle-treated rats; ###p<0.001 compared to vehicle/quinpirole-treated rats (Fisher post hoc test).
Effect of A-437203, a Selective D3 Receptor Antagonist, on Rat FST
A-437203, a selective D3 receptor antagonist, was initially tested alone in rat FST and results are expressed in Table 1. Doses of A-437203 evaluated were 0.52, 1.75, 5.24, and 17.46 μmol/kg i.p. Doses were chosen based on the selectivity of A-437203 for D3 vs D2 dopamine receptors (Chaperon et al, 2003) and reports indicating that the effects of A-437203 at doses of 17.46 μmol/kg (10 mg/kg) or lower are clearly mediated by D3 but not D2 receptors, since higher doses of the compound such as 174.6 μmol/kg (100 mg/kg) are necessary to bind and block D2 receptor from the irreversible inactivation induced by the alkylating agent EEDG (Drescher et al, 2002; Ramirez et al, 2003). ANOVA revealed no significant difference between the treatments for any of the behaviors analyzed (F4, 45=1.12, p=0.359 for immobility, F4, 45=0.188, p=0.943 for climbing, and F4, 45=1.634, p=0.182 for swimming). Based on these results, the dose of 17.46 μmol/kg i.p. of A-437203 was selected for further experiments.
Effect of A-437203 on the Effect of Quinpirole on Rat FST
As observed in previous experiments, administration of quinpirole induced a dose-dependent reduction in immobility time and an increase in climbing time. The D3 receptor antagonist A-437203 did not have an effect alone in the model; however, it showed a trend to attenuate the anti-immobility effect at the lower dose of 0.4 μmol/kg but not at the higher dose of 1.0 μmol/kg of quinpirole.
The effect of administration of the D3 receptor antagonist A-437203 on the effect of quinpirole in rat FST is shown in Figure 5. Two-way ANOVA for immobility time indicated a significant quinpirole effect (F2, 54=59.4318, p<0.001), a significant antagonist treatment (A-437203) effect (F1, 54=9.8622, p<0.01), but a nonsignificant antagonist × quinpirole interaction (F2, 54=2.4319, p=0.0974). While there was no significant interaction, it appears that A-437203 showed a nonsignificant trend to attenuate the quinpirole-induced antidepressant-like activity at the dose of quinpirole 0.4 μmol/kg dose (Figure 5). Two-way ANOVA for climbing revealed a significant quinpirole effect (F2, 54=48.3166, p<0.001), an almost significant antagonist treatment (A-437203) effect (F1, 54=3.8044, p=0.0563), and a nonsignificant antagonist × quinpirole interaction (F2, 54=0.2455, p=0.7831). The effect of quinpirole decreasing immobility and increasing climbing time in the model was revealed through the significant main effect of quinpirole and the collapsed data of each quinpirole dose (0.4 and 1.0 μmol/kg), as compared to vehicle (p<0.001). Statistical analysis for swimming indicated no significant effect for quinpirole, the antagonist or for the interaction.
Effect of A-437203 on the effect of quinpirole on rat FST. Graph represents mean±SEM for immobility, climbing, and swimming time during the 5-min test swim. A-437203 (17.46 μmol/kg i.p.) was administered 15 min before each quinpirole injection. Quinpirole (0.4, and 1.0 μmol/kg s.c.) was injected 24, 5, and 0.5 h before testing (n=10/group). No significant antagonist × quinpirole interaction was observed. Quinpirole induced a decrease in immobility and increase in climbing time that was revealed through a significant main effect of quinpirole and the collapsed data of each quinpirole dose (0.4 and 1.0 μmol/kg) as compared to vehicle (p<0.001).
Effect of L-745,870, a Selective D4 Antagonist, on Rat FST
Doses of L-745,870 were selected according to previous work reporting the behavioral profile of this compound (Bristow et al, 1997; Cao and Rodgers, 1997; Patel et al, 1997). ANOVA indicated no significant effect for any of the doses of L-745,870 in any of the parameters tested (F4, 43=1.867, p=0.134 for immobility time, F4, 43=1.593, p=0.193 for climbing time, and F4, 43=0.805, p=0.529 for swimming time). There was a trend towards an increase in immobility at the higher doses of L-745,870 (2.3 and 5.7 μmol/kg) as can be observed in Table 1. The dose of 1.15 μmol/kg of L-745,870 was therefore selected for the subsequent blockade study of quinpirole effects.
Effect of L-745,870 on the Effect of Quinpirole on Rat FST
Figure 6 shows the effects of pretreatment with the D4 receptor antagonist, L-745,870, on quinpirole-induced effects in rat FST. Two-way ANOVA for immobility time indicated a significant quinpirole effect (F2, 54=61.261, p<0.001), a nonsignificant antagonist treatment effect (F1, 54=0.115, p=0.736), and a nonsignificant antagonist × quinpirole interaction (F2, 54=0.7275, p=0.488). Statistical analysis for climbing time also revealed a similar result. Two-way ANOVA for climbing time showed a significant quinpirole effect (F2, 54=85.589, p<0.001), but a nonsignificant antagonist treatment effect (F1, 54=2.5619, p=0.1153), and a nonsignificant antagonist × quinpirole interaction (F2, 54=0.6278, p=0.538). The effect of quinpirole decreasing immobility and increasing climbing time in the model was revealed through the significant main effect of quinpirole and the collapsed data of each quinpirole dose (0.4 and 1.0 μmol/kg) as compared to vehicle (p<0.001). Two-way ANOVA for swimming time showed a significant quinpirole effect (F2, 54=5.995, p<0.01), but a nonsignificant antagonist treatment effect (F1, 54=1.837, p=0.181), and a nonsignificant antagonist × quinpirole interaction (F2, 54=0.017, p=0.983). Thus, quinpirole induced a decrease in immobility time and an increase in climbing time at both tested doses (0.4 and 1.0 μmol/kg). Administration of the D4 antagonist L-745,870 alone (1.15 μmol/kg) did not induce any change either in immobility, climbing, or swimming time compared to vehicle/vehicle control rats. Moreover, pretreatment with L-745,870 did not antagonize the effects of any of the doses of quinpirole in any of the parameters recorded.
Effect of L-745,870 on the effect of quinpirole on rat FST. Graph represents mean±SEM for immobility, climbing, and swimming time during the 5-min test swim. L-745,870 (1.15 μmol/kg i.p.) was administered 15 min before each quinpirole injection. Quinpirole (0.4 and 1.0 μmol/kg s.c.) was injected 24, 5, and 0.5 h before testing (n=10/group). No significant antagonist × quinpirole interaction was observed. Quinpirole induced a decrease in immobility and increase in climbing time that was revealed through a significant main effect of quinpirole and the collapsed data of each quinpirole dose (0.4 and 1.0 μmol/kg) as compared to vehicle (p<0.001).
Effect of Quinpirole on Locomotor Activity
Acute administration of quinpirole induced a biphasic effect in the locomotor activity assay, consisting of a significant reduction in total distance moved at the lowest dose of quinpirole (0.4 μmol/kg), but a significant increase in locomotion at higher doses (1.0 and 2.0 μmol/kg) during 90 min of testing (data not shown). Chronic administration of dopamine agonists, such as quinpirole, can result in behavioral sensitization to the activity response to the drug (Szechtman et al, 1993; Koeltzow et al, 2003; Lomanowska et al, 2004). Considering that subchronic treatment with quinpirole, similar to that used in FST (three injections in a 24-h period), might also be associated with induction of behavioral sensitization, rats were tested for spontaneous activity after three injections of quinpirole (0.4 and 1.0 μmol/kg=0.1 and 0.25 mg/kg) according to the same protocol treatment used for rat FST. Figure 7 shows the effect of subchronic quinpirole administration on spontaneous locomotor activity in rats and the time course of the activity effects induced by quinpirole. ANOVA for total distance moved indicated a significant treatment effect (F2, 27=5.6058, p<0.01). Subchronic treatment with quinpirole 0.4 μmol/kg did not increase locomotor activity compared to vehicle-treated rats, but quinpirole 1.0 μmol/kg induced a significant increase in activity (p<0.01). Activity data from quinpirole permit the differentiation of the anti-immobility effect observed in FST from general activation.
Effect of quinpirole a D2-like agonist on locomotor activity. Graph represents mean±SEM of total distance moved during 90 min (a) and time course of activity in 5-min intervals (b) following quinpirole administration (n=10/group). Locomotor activity was recorded after three quinpirole injections, following the same administration schedule used for rat FST (0.4 and 1.0 μmol/kg s.c. administered 25, 5, and 0 h before the test). **p<0.01 compared to vehicle-treated rats.
DISCUSSION
The present study supports the hypothesis that activation of D2-like receptors is associated with an antidepressant-like profile in rat FST. This detailed study assessed the involvement of specific dopamine D2-like receptors, and is the first to show that D2 and to a lesser extent D3, but not D4 receptors, are implicated in the modulation of depressive-like behaviors in a behavioral despair test, the FST. Nomifensine, a selective dopamine reuptake inhibitor that increases dopamine concentration in the synapses, and quinpirole, a dopamine D2-like receptor agonist, were associated with a significant decrease in immobility time and a significant increase in climbing behavior (Figures 1 and 2). The administration of a D3 preferential agonist, PD 128907, also induced a modest but significant decrease in immobility time and increase in climbing time at the highest dose tested. In contrast, the administration of selective D4 agonists, such as PD 168077 and CP 226269, did not induce significant behavioral changes in rat FST compared to vehicle control rats (Figure 3). However, since the availability of agonists showing true selectivity among receptor subtypes in the D2-like receptor family is limited, we sought to further test the involvement of different receptors by taking the complementary approach, that is, assessing the ability of D2, D3, and D4 selective antagonists to attenuate quinpirole-induced changes in the FST. The D2/3/4 receptor antagonist haloperidol, completely antagonized the effect of all doses of quinpirole in FST; however, the selective D3 receptor antagonist A-437203, only seemed to attenuate the effect of quinpirole at the low dose but not at the higher dose (Figures 4 and 5). In contrast, the selective D4 receptor antagonist L-745,870 did not attenuate any of the effects of quinpirole in rat FST (Figure 6). Thus, consistent with the agonist studies, results indicated there was blockade of quinpirole-induced antidepressant-like effects by a D2-like receptor antagonist, a trend towards a partial attenuation by a D3 receptor antagonist, but no effect of a D4 receptor antagonist. These findings suggest that an enhancement of dopamine function, and more specifically activation of dopamine D2 and, to a lesser extent, D3 receptors with dopamine agonists, may have relevance for the therapeutic treatment of depression.
The D3 receptor has been implicated in multiple neuropsychiatric disorders including depression and schizophrenia. The literature reports antidepressant effects for dopamine D2/D3 receptor agonists such as bromocriptine, piribedil, pramipexole, and roxindole (Post et al, 1978; Bouras and Bridges, 1982; Willner, 1983a; Grunder et al, 1993; Willner et al, 1994; Maj et al, 1996b; Corrigan et al, 2000). Pramipexole, a D3 receptor preferring agonist, has been reported to reverse the suppression of sucrose intake in animals chronically exposed to mild unpredictable stress, and showed efficacy in FST (Willner et al, 1994; Maj et al, 1997). Pramipexole has also demonstrated similar efficacy to fluoxetine for the treatment of major depressive disorder in a clinical trial (Corrigan et al, 2000). Within our study, the effects of the D3 preferential compound were modest but consistent; however, there remains the possibility that the effects of the D3 preferential compound were due to affinity for the D2 receptor. PD 128907 is a preferential D3 agonist, which shows preference for D3 vs D2 receptors in binding and functional studies using recombinant hD3 and hD2 receptors; however, the selectivity for D3 vs D2 is modest (14-fold). Thus, the finding that PD 128907 only induces a modest antidepressant-like effect at the highest dose tested may suggest that this dose is beginning to stimulate D2 receptors which are in turn mediating the antidepressant effect. However, the dose of PD 128907 that showed antidepressant-like activity in our study (0.7 μmol/kg=0.2 mg/kg; Figure 3) has been previously reported to be selective for stimulating D3 receptors suggesting that this modest effect may reflect a modest ability of D3 receptor activation to induce an antidepressant effect in FST (Bristow et al, 1996; Chaperon et al, 2003). Potential involvement of D3 receptors might also be indicated by the data obtained in the antagonism study. A-437203 is an antagonist with high affinity for D3 receptors and relatively high selectivity compared to other dopamine receptor subtypes (44-fold selective for D3 vs D2). Since quinpirole, a D2-like receptor agonist, shows some degree of selectivity for the D3 receptors (8.5-fold more selective for D3 over D2 receptors) (Levant et al, 1993), the finding that A-437203 tended to attenuate the lower but not the higher dose of quinpirole may suggest that the antagonist was capable of antagonizing the more D3-mediated effects of quinpirole, but unable to compete with the effect of the higher dose of quinpirole that fully stimulated D2 receptors (Figure 5). Thus, although D3 receptors do not appear to play as robust of a role as D2 receptors, they appear to contribute to this effect. However, further investigation is necessary to conclude whether this effect is specifically related with D3 receptors or due to nonspecific stimulation of dopamine receptors (ie D2 receptors).
Similar to D3 receptors, D4 receptors have been extensively investigated as a target for the treatment of schizophrenia. Interestingly, distribution of D4 receptors in brain areas associated with regulation of emotions and cognition such as prefrontal cortex and mesolimbic areas (Matsumoto et al, 1995; Ariano et al, 1997; Defagot and Antonelli, 1997; Defagot et al, 1997; Tarazi et al, 1997; Oak et al, 2000) also make them an intriguing candidate to play a role in depressive-like behaviors. However, using selective D4 agonists and antagonists, we found no indication of the involvement of D4 receptors in the FST. The marked selectivity of the D4 agonists (PD 168077, 100-fold selective vs D2 receptors and 200-fold selective vs D3 receptors; and CP 226269, 150-fold selective vs D2 receptors and 650-fold selective vs D3 receptors) (Moreland et al, 2004), and their lack of efficacy in the rat FST model, as well as for the D4 antagonist L-745,870 (10000-fold selective for D4 receptors vs D2 and D3) (Moreland et al, 2004), and the lack of efficacy blocking the effect of quinpirole in FST suggest that D4 receptors are clearly not involved in the mediation of antidepressant-like activity.
Since dopamine agonists like quinpirole can induce hyperactivity acutely as well as induce sensitization following repeated administration (Szechtman et al, 1993; Lomanowska et al, 2004), it could be proposed that the anti-immobility effects seen in the present study were merely the reflection of nonspecific locomotor activation. In order to address this, quinpirole was tested in a locomotor activity assay after a subchronic administration schedule identical to the administration regime used for rat FST. While there was a significant increase in locomotor activity levels following subchronic treatment with 1.0 μmol/kg of quinpirole, there was no change in locomotor activity with 0.4 μmol/kg. Thus, the effect of quinpirole in rat FST can be differentiated from general locomotor stimulation since antidepressant-like effects were clearly seen at doses not associated with hyperactivity (0.4 μmol/kg=0.1 mg/kg; Figure 2). In addition, potential increases in motor activity induced by stereotyped behavior do not seem likely since stereotypy usually occurs at higher doses than hyperlocomotion (Kurashima et al, 1995) and animals showed clear escape-oriented behaviors in the model (ie increase in climbing behavior). The effect observed in FST with PD 128907, as well as with low doses of nomifensine, can also be differentiated from general activation effect (data not shown).
Dopamine is believed to play a critical role in the neurobiology of depression and in the mechanism of action of antidepressant drugs, a hypothesis based on clinical and preclinical observations (Willner, 1983b; Fibiger, 1995; Charney, 1998; D'Aquila et al, 2000b). As stated above, compounds acting as agonists on dopamine D2/D3 receptors, such as bromocriptine, piribedil, pramipexole, and roxindole (Post et al, 1978; Bouras and Bridges, 1982; Willner, 1983a; Borsini et al, 1988; Muscat et al, 1992; Grunder et al, 1993; Willner et al, 1994; Maj et al, 1996b; Corrigan et al, 2000), and compounds that enhance dopamine levels inhibiting selective dopamine reuptake like amineptine, nomifensine, and bupropion (Kinney, 1985; Dalery et al, 1997), have shown antidepressant-like activity in both preclinical models and clinical settings. Interestingly, it has been proposed that an increase in dopamine activity induced by chronic antidepressant treatments is one of the mechanisms of action underlying the efficacy of these compounds, since their effects are antagonized by low doses of D2/D3 antagonists (Borsini et al, 1984; Borsini et al, 1985a, 1985b; Pulvirenti and Samanin, 1986; Sampson et al, 1991; Serra et al, 1992; D'Aquila et al, 2000a). Moreover, antidepressant treatments are associated with increased dopaminergic function in the mesolimbic system and increased behavioral response to dopamine agonists (Spyraki and Fibiger, 1981; Maj et al, 1984a, 1984b; Cervo and Samanin, 1987; De Montis et al, 1990; Ainsworth et al, 1998a; D'Aquila et al, 2000b, 2003), which takes place after chronic treatment (2–3 weeks), a period of time that correlates with that necessary for the onset of antidepressant activity in the clinic.
Dopaminergic deficits are associated with several psychiatric symptoms in man, such as depression (Kapur and Mann, 1992; Fibiger, 1995; Pania and Gessab, 2002). A deficiency of mesolimbic dopaminergic pathway plays a critical role for the etiology of symptoms of depression like anhedonia and decreased motivation (Willner, 1983b; Schultz, 1997; Berridge and Robinson, 1998; Naranjo et al, 2001). Mesolimbic dopamine projections are a crucial component in the neural circuitry of reward and/or incentive motivation, both dysfunctional in major depression disorder (Fibiger, 1995; Naranjo et al, 2001). Dopaminergic abnormalities such as reduced dopamine transporter and upregulation of D2/D3 receptors have been found in amygdaloid nuclei in postmortem brain of major depressed patients (Klimek et al, 2002) as well as reduced concentration of homovalinic acid, a dopamine metabolite, in cerebrospinal fluid (Willner, 1983b) and plasma of depressed patients (Lambert et al, 2000). Furthermore, administration of dopamine antagonists as well as drugs that produce depletion of catecholamines (ie reserpine) can elicit symptoms in healthy volunteers that resemble those described in depression such as anhedonia, and lack of volition and energy (Wise et al, 1978; Willner, 1983a), suggesting that reduction in dopamine transmission is an important neurochemical substrate associated with depression. Studies with schizophrenic patients treated with antipsychotics indicate that they may experience common side effects such as neuroleptic dysphoria (Weiden et al, 1989; Voruganti and Awad, 2004), negative affective states and depressive symptoms (Harrow et al, 1994). Moreover, D2 receptor blockade associated with typical antipsychotic treatment directly correlates with and contributes to the emergence of severe depressive symptoms in schizophrenic patients (Bressan et al, 2002; de Haan et al, 2004). Increased immobility in rat FST is associated with a depressive-like state in the FST. In the present study, we did not see that kind of effect by dopamine antagonists at the doses tested in the animal model (Table 1). However, it is well known that antipsychotics induce hypolocomotion at high doses, and despite the sedative effects of these compounds this behavior could be representative of depressive-like states (Porsolt et al, 1978).
In summary, dopamine receptor agonists, especially dopamine D2/D3 receptor agonists, could be potentially effective for the treatment of depression (Forrest et al, 1977; Bouras and Bridges, 1982; Lopez-Ibor Alino et al, 1982; Kinney, 1985; Corrigan et al, 2000). However, given the implication of the D2 receptor in reward and addiction, one would have to clearly separate antidepressant activity from the risk for potential abuse (Caine et al, 1997; Haddad, 1999; Ellinwood et al, 2002; Rouge-Pont et al, 2002; Farvolden et al, 2003). Furthermore, activation of dopamine function and/or supersensitivity of dopaminergic neurotransmission may play an important role in the induction of mania episodes in patients with bipolar disorders (D'Aquila et al, 2003), another obstacle for the use of D2 receptor agonists for the treatment of depression (Massat et al, 2002; Yatham, 2002; Serretti et al, 2004). Mood changes observed in bipolar patients from mania to depression are critical events in the course of the disorder and might depend upon parallel changes in mesolimbic dopamine system sensitivity. Finally, the understanding of the neurobiological basis of depression, including the importance of the dopamine system, will be important in the development of new treatments for depression with better efficacy and tolerability, faster onset of action and fewer side effects.
References
Ainsworth K, Smith SE, Sharp T (1998a). Repeated administration of fluoxetine, desipramine and tranylcypromine increases dopamine D2-like but not D1-like receptor function in the rat. J Psychopharmacol 12: 252–257.
Ainsworth K, Smith SE, Zetterstrom TS, Pei Q, Franklin M, Sharp T (1998b). Effect of antidepressant drugs on dopamine D1 and D2 receptor expression and dopamine release in the nucleus accumbens of the rat. Psychopharmacology (Berlin) 140: 470–477.
Ariano MA, Wang J, Noblett KL, Larson ER, Sibley DR (1997). Cellular distribution of the rat D4 dopamine receptor protein in the CNS using anti-receptor antisera. Brain Res 752: 26–34.
Berridge KC, Robinson TE (1998). What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev 28: 309–369.
Borsini F, Lecci A, Mancinelli A, D'Aranno V, Meli A (1988). Stimulation of dopamine D-2 but not D-1 receptors reduces immobility time of rats in the forced swimming test: implication for antidepressant activity. Eur J Pharmacol 148: 301–307.
Borsini F, Nowakowska E, Pulvirenti L, Samanin R (1985a). Repeated treatment with amitriptyline reduces immobility in the behavioural ‘despair’ test in rats by activating dopaminergic and beta-adrenergic mechanisms. J Pharm Pharmacol 37: 137–138.
Borsini F, Nowakowska E, Samanin R (1984). Effect of repeated treatment with desipramine in the behavioral ‘despair’ test in rats: antagonism by ‘atypical’ but not ‘classical’ neuroleptics or antiadrenergic drugs. Life Sci 34: 1171–1176.
Borsini F, Pulvirenti L, Samanin R (1985b). Evidence of dopamine involvement in the effect of repeated treatment with various antidepressants in the behavioural ‘despair’ test in rats. Eur J Pharmacol 110: 253–256.
Bouras N, Bridges PK (1982). Bromocriptine in depression. Curr Med Res Opin 8: 150–153.
Bressan RA, Costa DC, Jones HM, Ell PJ, Pilowsky LS (2002). Typical antipsychotic drugs—D(2) receptor occupancy and depressive symptoms in schizophrenia. Schizophr Res 56: 31–36.
Bristow LJ, Collinson N, Cook GP, Curtis N, Freedman SB, Kulagowski JJ et al (1997). L-745,870, a subtype selective dopamine D4 receptor antagonist, does not exhibit a neuroleptic-like profile in rodent behavioral tests. J Pharmacol Exp Ther 283: 1256–1263.
Bristow LJ, Cook GP, Gay JC, Kulagowski JJ, Landon L, Murray F et al (1996). The behavioural and neurochemical profile of the putative dopamine D3 receptor agonist, (+)-PD 128907, in the rat. Neuropharmacology 35: 285–294.
Caine SB, Koob GF, Parsons LH, Everitt BJ, Schwartz JC, Sokoloff P (1997). D3 receptor test in vitro predicts decreased cocaine self-administration in rats. Neuroreport 8: 2373–2377.
Cao BJ, Rodgers RJ (1997). Dopamine D4 receptor and anxiety: behavioural profiles of clozapine, L-745,870 and L-741,742 in the mouse plus-maze. Eur J Pharmacol 335: 117–125.
Cervo L, Samanin R (1987). Evidence that dopamine mechanisms in the nucleus accumbens are selectively involved in the effect of desipramine in the forced swimming test. Neuropharmacology 26: 1469–1472.
Chaperon F, Tricklebank MD, Unger L, Neijt HC (2003). Evidence for regulation of body temperature in rats by dopamine D2 receptor and possible influence of D1 but not D3 and D4 receptors. Neuropharmacology 44: 1047–1053.
Charney DS (1998). Monoamine dysfunction and the pathophysiology and treatment of depression. J Clin Psychiatry 59 (Suppl 14): 11–14.
Corrigan MH, Denahan AQ, Wright CE, Ragual RJ, Evans DL (2000). Comparison of pramipexole, fluoxetine, and placebo in patients with major depression. Depress Anxiety 11: 58–65.
Dalery J, Rochat C, Peyron E, Bernard G (1997). The efficacy and acceptability of amineptine versus fluoxetine in major depression. Int Clin Psychopharmacol 12 (Suppl 3): S35–S38.
D'Aquila PS, Collu M, Gessa GL, Serra G (2000a). The role of dopamine in the mechanism of action of antidepressant drugs. Eur J Pharmacol 405: 365–373.
D'Aquila PS, Peana AT, Carboni V, Serra G (2000b). Different effect of desipramine on locomotor activity in quinpirole-treated rats after repeated restraint and chronic mild stress. J Psychopharmacol 14: 347–352.
D'Aquila PS, Peana AT, Panin F, Grixoni C, Cossu M, Serra G (2003). Reversal of antidepressant-induced dopaminergic behavioural supersensitivity after long-term chronic imipramine withdrawal. Eur J Pharmacol 458: 129–134.
de Haan L, Lavalaye J, van Bruggen M, van Nimwegen L, Booij J, van Amelsvoort T et al (2004). Subjective experience and dopamine D2 receptor occupancy in patients treated with antipsychotics: clinical implications. Can J Psychiatry 49: 290–296.
De Montis GM, Devoto P, Gessa GL, Meloni D, Porcella A, Saba P et al (1990). Central dopaminergic transmission is selectively increased in the limbic system of rats chronically exposed to antidepressants. Eur J Pharmacol 180: 31–35.
Defagot MC, Antonelli MC (1997). Autoradiographic localization of the putative D4 dopamine receptor in rat brain. Neurochem Res 22: 401–407.
Defagot MC, Malchiodi EL, Villar MJ, Antonelli MC (1997). Distribution of D4 dopamine receptor in rat brain with sequence-specific antibodies. Brain Res Mol Brain Res 45: 1–12.
Detke MJ, Rickels M, Lucki I (1995). Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berlin) 121: 66–72.
Drescher KU, Garcia-Ladona FJ, Teschendorf HJ, Traut M, Unger L, Wicke KM et al (2002). In vivo effects of the selective dopamine D3 receptor antagonist A-437203. Program No. 894.6. Abstract Viewer/Itinerary Planner. Society for Neuroscience, Washington, DC; 2002. Online.
Duterte-Boucher D, Leclere JF, Panissaud C, Costentin J (1988). Acute effects of direct dopamine agonists in the mouse behavioral despair test. Eur J Pharmacol 154: 185–190.
Ellinwood EH, Davidson C, Yu GZ, King GR, Lee TH (2002). Effect of daily dosing duration of direct and indirect dopamine receptor agonists: cocaine cross-tolerance following chronic regimens. Eur Neuropsychopharmacol 12: 407–415.
Farvolden P, Kennedy SH, Lam RW (2003). Recent developments in the psychobiology and pharmacotherapy of depression: optimising existing treatments and novel approaches for the future. Expert Opin Investig Drugs 12: 65–86.
Fibiger HC (1995). Neurobiology of depression: focus on dopamine. Adv Biochem Psychopharmacol 49: 1–17.
Forrest A, Hewett A, Nicholson P (1977). Controlled randomized group comparison of nomifensine and imipramine in depressive illness. Br J Clin Pharmacol 4 (Suppl 2): 215S–220S.
Gambarana C, Ghiglieri O, Tagliamonte A, D'Alessandro N, de Montis MG (1995). Crucial role of D1 dopamine receptors in mediating the antidepressant effect of imipramine. Pharmacol Biochem Behav 50: 147–151.
Grunder G, Wetzel H, Hammes E, Benkert O (1993). Roxindole, a dopamine autoreceptor agonist, in the treatment of major depression. Psychopharmacology (Berlin) 111: 123–126.
Haddad P (1999). Do antidepressants have any potential to cause addiction? J Psychopharmacol 13: 300–307.
Harrow M, Yonan CA, Sands JR, Marengo J (1994). Depression in schizophrenia: are neuroleptics, akinesia, or anhedonia involved? Schizophr Bull 20: 327–338.
Kapur S, Mann JJ (1992). Role of the dopaminergic system in depression. Biol Psychiatry 32: 1–17.
Kinney JL (1985). Nomifensine maleate: a new second-generation antidepressant. Clin Pharm 4: 625–636.
Klimek V, Schenck JE, Han H, Stockmeier CA, Ordway GA (2002). Dopaminergic abnormalities in amygdaloid nuclei in major depression: a postmortem study. Biol Psychiatry 52: 740–748.
Koeltzow TE, Austin JD, Vezina P (2003). Behavioral sensitization to quinpirole is not associated with increased nucleus accumbens dopamine overflow. Neuropharmacology 44: 102–110.
Koob GF (1996). Hedonic valence, dopamine and motivation. Mol Psychiatry 1: 186–189.
Kurashima M, Yamada K, Nagashima M, Shirakawa K, Furukawa T (1995). Effects of putative dopamine D3 receptor agonists, 7-OH-DPAT, and quinpirole, on yawning, stereotypy, and body temperature in rats. Pharmacol Biochem Behav 52: 503–508.
Lambert G, Johansson M, Agren H, Friberg P (2000). Reduced brain norepinephrine and dopamine release in treatment-refractory depressive illness: evidence in support of the catecholamine hypothesis of mood disorders. Arch Gen Psychiatry 57: 787–793.
Levant B, Grigoriadis DE, DeSouza EB (1993). [3H]quinpirole binding to putative D2 and D3 dopamine receptors in rat brain and pituitary gland: a quantitative autoradiographic study. J Pharmacol Exp Ther 264: 991–1001.
Lomanowska A, Gormley S, Szechtman H (2004). Presynaptic stimulation and development of locomotor sensitization to the dopamine agonist quinpirole. Pharmacol Biochem Behav 77: 617–622.
Lopez-Ibor Alino JJ, Ayuso Gutierez JL, Montejo Iglesias ML, Ramons JL (1982). A double-blind clinical comparison between nomifensine and amitriptyline in the treatment of endogenous depressions. Int Pharmacopsychiatry 17 (Suppl 1): 97–105.
Maj J, Dziedzicka-Wasylewska M, Rogoz R, Rogoz Z (1998a). Effect of antidepressant drugs administered repeatedly on the dopamine D3 receptors in the rat brain. Eur J Pharmacol 351: 31–37.
Maj J, Dziedzicka-Wasylewska M, Rogoz R, Rogoz Z, Skuza G (1996a). Antidepressant drugs given repeatedly change the binding of the dopamine D2 receptor agonist, [3H]N-0437, to dopamine D2 receptors in the rat brain. Eur J Pharmacol 304: 49–54.
Maj J, Kolodziejczyk K, Rogoz Z, Skuza G (1996b). Roxindole, a potential antidepressant. I. Effect on the dopamine system. J Neural Transm Gen Sect 103: 627–641.
Maj J, Papp M, Skuza G, Bigajska K, Zazula M (1989). The influence of repeated treatment with imipramine, (+)- and (−)-oxaprotiline on behavioural effects of dopamine D-1 and D-2 agonists. J Neural Transm 76: 29–38.
Maj J, Rogoz Z, Skuza G, Kolodziejczyk K (1997). Antidepressant effects of pramipexole, a novel dopamine receptor agonist. J Neural Transm 104: 525–533.
Maj J, Rogoz Z, Skuza G, Margas W (1998b). Repeated trimipramine induces dopamine D2/D3 and alpha1-adrenergic up-regulation. J Neural Transm 105: 329–342.
Maj J, Rogoz Z, Skuza G, Sowinska H (1984a). Repeated treatment with antidepressant drugs increases the behavioural response to apomorphine. J Neural Transm 60: 273–282.
Maj J, Rogoz Z, Skuza G, Sowinska H (1984b). Repeated treatment with antidepressant drugs potentiates the locomotor response to (+)-amphetamine. J Pharm Pharmacol 36: 127–130.
Massat I, Souery D, Del-Favero J, Van Gestel S, Serretti A, Macciardi F et al (2002). Positive association of dopamine D2 receptor polymorphism with bipolar affective disorder in a European Multicenter Association Study of affective disorders. Am J Med Genet 114: 177–185.
Matsumoto M, Hidaka K, Tada S, Tasaki Y, Yamaguchi T (1995). Full-length cDNA cloning and distribution of human dopamine D4 receptor. Brain Res Mol Brain Res 29: 157–162.
Meiergerd SM, Schenk JO (1994). Kinetic evaluation of the commonality between the site(s) of action of cocaine and some other structurally similar and dissimilar inhibitors of the striatal transporter for dopamine. J Neurochem 63: 1683–1692.
Moreland RB, Nakane M, Donnelly-Roberts DL, Miller LN, Chang R, Uchic ME et al (2004). Comparative pharmacology of human dopamine D(2)-like receptor stable cell lines coupled to calcium flux through Galpha(qo5). Biochem Pharmacol 68: 761–772.
Muscat R, Papp M, Willner P (1992). Antidepressant-like effects of dopamine agonists in an animal model of depression. Biol Psychiatry 31: 937–946.
Naranjo CA, Tremblay LK, Busto UE (2001). The role of the brain reward system in depression. Prog Neuropsychopharmacol Biol Psychiatry 25: 781–823.
Nelson JC, Charney DS (1981). The symptoms of major depressive illness. Am J Psychiatry 138: 1–13.
Nunes Junior GP, Tufik S, Nobrega JN (1994). Autoradiographic analysis of D1 and D2 dopaminergic receptors in rat brain after paradoxical sleep deprivation. Brain Res Bull 34: 453–456.
Oak JN, Oldenhof J, Van Tol HH (2000). The dopamine D(4) receptor: one decade of research. Eur J Pharmacol 405: 303–327.
Pania L, Gessab GL (2002). Dopaminergic deficit and mood disorders. Int Clin Psychopharmacol 17 (Suppl 4): S1–S7 discussion S7.
Patel S, Freedman S, Chapman KL, Emms F, Fletcher AE, Knowles M et al (1997). Biological profile of L-745,870, a selective antagonist with high affinity for the dopamine D4 receptor. J Pharmacol Exp Ther 283: 636–647.
Porsolt RD, Anton G, Blavet N, Jalfre M (1978). Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol 47: 379–391.
Porsolt RD, Bertin A, Blavet N, Deniel M, Jalfre M (1979). Immobility induced by forced swimming in rats: effects of agents which modify central catecholamine and serotonin activity. Eur J Pharmacol 57: 201–210.
Post RM, Gerner RH, Carman JS, Gillin JC, Jimerson DC, Goodwin FK et al (1978). Effects of a dopamine agonist piribedil in depressed patients: relationship of pretreatment homovanillic acid to antidepressant response. Arch Gen Psychiatry 35: 609–615.
Pulvirenti L, Samanin R (1986). Antagonism by dopamine, but not noradrenaline receptor blockers of the anti-immobility activity of desipramine after different treatment schedules in the rat. Pharmacol Res Commun 18: 73–80.
Ramirez AD, Menniti FS, Wong SK, Shrikhande A (2003). Inactivation of dopamine receptors by EEDQ in vivo: Selective protection by D2/D3 compounds. Program No. 409.2. Abstract Viewer/Itinerary Planner. Society for Neuroscience, Washington, DC; 2003. Online.
Robbins TW, Everitt BJ (1996). Neurobehavioural mechanisms of reward and motivation. Curr Opin Neurobiol 6: 228–236.
Rogoz R, Dziedzicka-Wasylewska M (1999). Effects of antidepressant drugs on the dopamine D2/D3 receptors in the rat brain differentiated by agonist and antagonist binding—an autoradiographic analysis. Naunyn Schmiedebergs Arch Pharmacol 359: 178–186.
Rouge-Pont F, Usiello A, Benoit-Marand M, Gonon F, Piazza PV, Borrelli E (2002). Changes in extracellular dopamine induced by morphine and cocaine: crucial control by D2 receptors. J Neurosci 22: 3293–3301.
Sampson D, Willner P, Muscat R (1991). Reversal of antidepressant action by dopamine antagonists in an animal model of depression. Psychopharmacology (Berlin) 104: 491–495.
Schultz W (1997). Dopamine neurons and their role in reward mechanisms. Curr Opin Neurobiol 7: 191–197.
Serra G, Collu M, D'Aquila PS, De Montis GM, Gessa GL (1990). Possible role of dopamine D1 receptor in the behavioural supersensitivity to dopamine agonists induced by chronic treatment with antidepressants. Brain Res 527: 234–243.
Serra G, Collu M, D'Aquila PS, Gessa GL (1992). Role of the mesolimbic dopamine system in the mechanism of action of antidepressants. Pharmacol Toxicol 71 (Suppl 1): 72–85.
Serretti A, Artioli P, Zanardi R, Lorenzi C, Rossini D, Cusin C et al (2004). Genetic features of antidepressant induced mania and hypo-mania in bipolar disorder. Psychopharmacology (Berlin) 174: 504–511.
Sokoloff P, Andrieux M, Besancon R, Pilon C, Martres MP, Giros B et al (1992). Pharmacology of human dopamine D3 receptor expressed in a mammalian cell line: comparison with D2 receptor. Eur J Pharmacol 225: 331–337.
Spyraki C, Fibiger HC (1981). Behavioural evidence for supersensitivity of postsynaptic dopamine receptors in the mesolimbic system after chronic administration of desipramine. Eur J Pharmacol 74: 195–206.
Szechtman H, Talangbayan H, Eilam D (1993). Environmental and behavioral components of sensitization induced by the dopamine agonist quinpirole. Behav Pharmacol 4: 405–410.
Tarazi FI, Kula NS, Baldessarini RJ (1997). Regional distribution of dopamine D4 receptors in rat forebrain. Neuroreport 8: 3423–3426.
Unger L, Garcia-Ladona FJ, Wernet W, Sokoloff P, Wicke KM, Gross G (2002). In vitro characterization of the selective dopamine D3 receptor antagonist A-437203. Program No. 894.5. Abstract Viewer/Itinerary Planner. Society for Neuroscience, Washington, DC; 2002. Online.
Voruganti L, Awad AG (2004). Neuroleptic dysphoria: towards a new synthesis. Psychopharmacology (Berlin) 171: 121–132.
Weiden PJ, Mann JJ, Dixon L, Haas G, DeChillo N, Frances AJ (1989). Is neuroleptic dysphoria a healthy response? Compr Psychiatry 30: 546–552.
Willner P (1983a). Dopamine and depression: a review of recent evidence. I. Emperical studies. Brain Res 287: 211–224.
Willner P (1983b). Dopamine and depression: a review of recent evidence. II. Theoretical approaches. Brain Res 287: 225–236.
Willner P, Lappas S, Cheeta S, Muscat R (1994). Reversal of stress-induced anhedonia by the dopamine receptor agonist, pramipexole. Psychopharmacology (Berlin) 115: 454–462.
Willner P, Montgomery T (1981). Behavioural changes during withdrawal from desmethylimipramine (DMI). I. Interactions with amphetamine. Psychopharmacology (Berlin) 75: 54–59.
Wise RA, Spindler J, deWit H, Gerberg GJ (1978). Neuroleptic-induced ‘anhedonia’ in rats: pimozide blocks reward quality of food. Science 201: 262–264.
Yatham LN (2002). The role of novel antipsychotics in bipolar disorders. J Clin Psychiatry 63 (Suppl 3): 10–14.
Acknowledgements
This research has been funded by Abbott Laboratories.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Basso, A., Gallagher, K., Bratcher, N. et al. Antidepressant-Like Effect of D2/3 Receptor-, but not D4 Receptor-Activation in the Rat Forced Swim Test. Neuropsychopharmacol 30, 1257–1268 (2005). https://doi.org/10.1038/sj.npp.1300677
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1300677
Keywords
This article is cited by
-
Behavioural effects of APH199, a selective dopamine D4 receptor agonist, in animal models
Psychopharmacology (2023)
-
Nicotine preference and affective behavior of Cd81 knockout mice
Psychopharmacology (2021)
-
Simvastatin Therapy in the Acute Stage of Traumatic Brain Injury Attenuates Brain Trauma-Induced Depression-Like Behavior in Rats by Reducing Neuroinflammation in the Hippocampus
Neurocritical Care (2017)
-
Implication of dopamine D3 receptor activation in the reversion of Parkinson’s disease-related motivational deficits
Translational Psychiatry (2014)
-
Pre-gestational stress alters stress-response of pubertal offspring rat in sexually dimorphic and hemispherically asymmetric manner
BMC Neuroscience (2013)