Abstract
The acute influence of ethanol on cerebral activity induces complex psycho-physiological effects that are considerably more pronounced during acute ethanol influx than during maximal blood alcohol concentration (elimination phase). Despite the psychiatric and forensic relevance of these different ethanol effects, the underlying neuronal mechanisms are still unclear. In total, 20 male healthy volunteers were investigated each with three different experimental conditions in a randomized order using an intravenous ethanol challenge (40 g bolus infusion): during influx phase, elimination phase, and under placebo condition. During and after the ethanol (or placebo) infusion, neuropsychological testing of divided attention for visual and auditory stimuli was performed with subsequent 18-FDG PET acquisition. The PET data were analysed using SPM99. Ethanol influx and elimination phase showed focal activations in the bilateral striatum and frontal cortex and deactivations in the occipital cortex. The comparison of influx phase vs elimination phase revealed activations in the anterior cingulate and right prefrontal cortex, relevant deactivations were found in the left superior temporal cortex including Wernicke's area. Neuropsychological testing showed an attentional impairment under ethanol influx compared to ethanol elimination and placebo with an inverse correlation of the attentional performance for auditory stimuli to occipital activity and for visual stimuli to the left temporal (including auditory) cortex. Acute ethanol administration in healthy volunteers stimulates those striatal regions that are considered to have a particular relevance for alcohol craving (‘reward system’). Modality specific reciprocal inhibition of sensory cortex activity seems to be relevant for attentional performance during acute alcohol impact.
Similar content being viewed by others
INTRODUCTION
Although alcohol, having considerable medical and epidemiological relevance, is the most common ‘drug’ taken throughout the world, the neurophysiological processes underlying the psychotropic effects of acute ethanol intake are still unclear.
It is well known from clinical and forensic observations of acute ethanol effects that the complex psychophysiological changes during the acute cerebral ethanol uptake (‘influx phase’) is distinctive from those which occur after the peak of blood alcohol concentration is reached (‘elimination phase’), a phenomenon that was first described by Mellanby (1919). Early neuropsychological examinations under experimental alcohol intoxication revealed that performance deficits are more pronounced during the influx phase compared to the elimination phase, which led to the assumption that the maximal psychotropic alcohol effect precedes the maximal venous blood alcohol concentration (Bschor, 1952). Despite these early observations and the psychiatric and forensic importance of the different alcohol effects during influx and elimination, the neuronal mechanisms during the different phases of ethanol kinetics have not yet been thoroughly investigated.
The few functional imaging studies on acute alcohol effects published so far revealed neuronal activations in the right prefrontal cortex (Tiihonen et al, 1994), in the left temporal cortex and in left striatal regions (Wang et al, 2000) and the bilateral thalamus (Boecker et al, 1996), whereas decreases were found in the occipital cortex (Volkow et al, 1990; Wang et al, 2000) and in the bilateral cerebellum (Boecker et al, 1996).
First investigations combining positron emission tomography (PET) with [C-11]ethanol and magnetic resonance spectroscopy yielded that during the influx phase there is a high [C-11]ethanol uptake in the basal ganglia independently from regional cerebral blood flow (Amberg et al, 2002).
The majority of the investigations on acute alcohol impact on cerebral blood flow or cerebral glucose metabolism used oral alcohol administration in order to simulate ‘physiological’ uptake conditions (de Wit et al, 1990; Volkow et al, 1990; Schwartz et al, 1993; Boecker et al, 1996; Wang et al, 2000). The principle problems of this approach are (1) high interindividual variability of alcohol uptake by ingestion that impedes comparable arterial influx conditions and (2) impossibility to induce a fast arterial influx by means of ‘normal’ oral alcohol doses. Therefore, despite its ‘unphysiological’ character, an intravenous alcohol administration would enable a very similar arterial input and would provide standardized/comparable uptake conditions even measured over multiple subjects.
It was the purpose of this study to investigate the cerebral neuronal correlates of the different phases of ethanol kinetics compared to placebo by means of [F-18]fluoro-deoxyglucose (18-FDG) PET.
METHODS
This study was carried out following the Helsinki Declaration and was approved by the local ethics committee, the Federal Health Administration (BfArM) and the radiation protection authorities (BfS).
Subjects
In total, 20 healthy male volunteers (range: 23–31 years) were included in this study. The subjects had no current or previous history of relevant physical illness, no current or past psychiatric disorders, no family history of major psychiatric disorder in first-degree relatives, and they were not regularly taking medication. All of the subjects, who were students from our medical school, were classified as ‘social drinkers’, who did not exceed a maximal mean alcohol intake of 30 g ethanol per day. Before entering the study, subjects were screened with a standard psychiatric interview (CIDI: Composite International Diagnostic Interview), a medical history, and a clinical examination including drug screening. All subjects gave written informed consent.
Activation Protocol
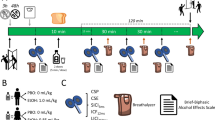
In a single-blinded study design, each subject underwent three PET scans with 150±20 MBq [F-18]fluoro-deoxyglucose (18-FDG) and receiving 1 × placebo and 2 × ethanol infusion in a randomized order within a time interval of 4 weeks. All examinations were performed at the same time of day (all in the am, for the same subject time of injection within 1 h) to minimize influences by circadian variability of cerebral glucose metabolism (Bartlett et al, 1988) or alcohol sensitivity.
On the examination day, subjects were asked to fast for at least 8 h before the PET study but had free access to water. The last time of alcohol consumption had to be at least 24 h ago. An indwelling cannula was placed in the veins of both lower arms for subsequent blood samplings. Placebo (500 ml 0.9% saline solution) and alcohol (40 g ethanol in 500 ml 0.9% saline solution) were administered intravenously while the subjects were instructed to relax with eyes closed lying in a quiet and darkened room. Vital parameters were taken throughout the entire experiment. Neuropsychological testing was performed at different times before and during the ethanol challenge (see below) but not after the 18-FDG injection in order to avoid sensory or cognitive stimulation by the testing procedure during the relevant cerebral 18-FDG uptake phase (0–30 min p.i.). Blood alcohol concentrations were measured before the onset of the infusion and 15 (at the end of the influx phase: see below), 30, 45 and 60 min after the start of the ethanol infusion.
Ethanol Influx Condition
Ethanol infusion was administered over 15 min with the 18-FDG bolus at the beginning of the infusion. Neuropsychological testing was performed 5 min before ethanol infusion.
Ethanol Elimination Condition
Ethanol infusion was administered over 15 min with the 18-FDG bolus 25 min after the end of the ethanol infusion. Neuropsychological testing was performed 5 min before ethanol infusion, 5 min after the beginning of the infusion (representing acute influx conditions), and 15 min after the end of the infusion (representing elimination conditions).
Placebo Condition
Placebo infusion was administered over 15 min with the 18-FDG bolus at the beginning of the infusion. Neuropsychological testing was performed 5 min before placebo infusion.
Neuropsychological Testing
For the neuropsychological testing, two subtests of the computerized Testbattery for Attentional Performance (TAP 1.7; Zimmermann and Fimm, 2002) have been applied. The Divided Attention task uses visuo-spatial and auditory stimulation simultaneously.
The visual task was to detect squares made up by four crosses out of eight crosses that appear in 100 random configurations on the computer screen. The parallel acoustical task requires detecting irregularities in the alternating sequence of 200 high and low beeps. Outcome parameters are total reaction time (median), reaction time for visual and auditory stimuli (median) number of errors and omissions.
The subtest Flexibility requires focusing attention and shifting focus repeatedly between two sets of stimuli: numbers and letters. For 100 times, a letter and a number are presented simultaneously and randomly on the left or the right side of the fixation point on the computer screen. The test requires pressing the button either on the side where the number appears or where the letter appears. With each presentation the target changes from ‘number’ to ‘letter’. Outcome parameters are reaction time (median), number of errors, and omissions.
Both subtests were processed repeatedly by every participant under the different experimental conditions. Training effects on test performance were controlled by the random application of the different conditions (placebo, elimination condition, influx condition).
PET Examination and Image Reconstruction
PET examinations were performed using an ECAT Exact PET Scanner (Siemens/CTI, Knoxville). The emission scan started 30 min after 18-FDG injection and continued for 20 min in a three-dimensional acquisition mode for one bed position (axial field of view of 16.2 cm). Attenuation correction was performed using a computerized threshold limit routine to define an isodensity contour for the maximum cerebral activity/pixel. The exact position of these isodensity contours was controlled visually slice-by-slice and eventually corrected manually. In all, 47 transversal slices (slice thickness 3.375 mm) were reconstructed using filtered back projection with a 4 mm Hamming filter. The transaxial resolution was 6.0 mm FWHM (full-width at half-maximum).
Absolute Quantification of Cerebral Glucose Metabolism
Absolute glucose consumption rate (rMRGlu) was calculated for each pixel according to the autoradiography method after Sokoloff et al (1977) and Phelps et al (1979) using measured input function, tissue radioactivity concentration, and blood glucose concentration. For quantification of grey matter glucose metabolism, a set of rate constants K1–k4 and a lumped constant were used (Phelps et al, 1979; Reivich et al, 1985). Cortical Regions-of-Interest were manually drawn to calculate the mean cortical rMRGlu for each condition (influx, elimination, placebo).
Statistical Analysis
The differences of the mean absolute cortical glucose metabolism (rMRGlu) in terms of μmol/min/100 g during influx, elimination, and placebo were compared using the paired t-test. The further analysis of the PET data was performed by means of Statistical Parametric Mapping software (SPM 99, Wellcome Department of Cognitive Neurology, London). Due to the high intra- and interindividual variability of the absolute cerebral glucose utilization, normalized tissue activities (proportional scaling: see below) were used for the SPM analysis.
The PET images were realigned and stereotactically normalized into the standard anatomical space (Talairach and Tournoux, 1988) by means of linear and nonlinear transformation (Friston et al, 1995a). Subsequently, the normalized images were smoothed with a three-dimensional Gaussian filter using a 12 mm FWHM kernel, voxel size 2 × 2 × 2 mm3. The effect of the three different conditions (placebo/ethanol influx phase/ethanol elimination phase) on regional cerebral 18-FDG activity was estimated according to the general linear model (Friston et al, 1995b).
After proportional scaling of all PET scans to a mean global cerebral activity (Friston et al, 1990), t-statistical parametric maps (SPM {t}) were calculated. For the categorical comparisons (ethanol influx phase vs placebo, ethanol elimination phase vs placebo, ethanol influx phase vs ethanol elimination phase), the t-values of the scans were calculated on a voxel-by-voxel basis using a poled variance estimated from the whole-brain grey matter (Worsley et al, 1992). Since the resulting t-map is known to approximate closely to a standard Gaussian distribution (Worsley et al, 1992), the values were described as Z-scores. In order to determine a threshold for significant activation on the resulting t-map, the image smoothness was calculated (Friston et al, 1991), and a statistical threshold was estimated at a probability of p<0.00001. Resulting activation foci were considered significant, if they met a threshold of p<0.05, corrected for multiple comparisons across the whole-brain volume, based on the theory of random Gaussian fields (Lumer et al, 1998; Worsley et al, 1998).
The final step of the data analysis was the investigation of the functional correlation between the neuropsychological test performance and cortical metabolic activity. Those neuropsychological parameters (reaction time, number of errors, omissions), which were significantly reduced during ethanol influx or elimination, were correlated as an external covariate to the corresponding cortical metabolic data on a voxel-wise basis using a statistical threshold of p<0.001 (uncorrected) and a minimal cluster size >80 voxels (Bartenstein et al, 1997; Siebner et al, 2001).
As the image data were stereotactically normalized, maxima of the activation foci were reported with the respective stereotactic coordinates.
RESULTS
Absolute Cortical Glucose Metabolism
The mean cortical rMRGlu was significantly reduced (p<0.0001) during the ethanol influx vs placebo (29.7 vs 39.2 μmol/min/100 g) and the ethanol elimination vs placebo (29.2 vs 39.2 μmol/min/100 g), respectively. The very slight difference of the mean cortical rMRGlu between ethanol influx and ethanol elimination was not significant (p=0.744).
Ethanol Influx vs Placebo
The categorical comparison of neuronal metabolic changes during ethanol influx vs placebo revealed a significantly increased regional cerebral glucose metabolism above the given threshold of p<0.05 (corrected for multiple comparisons) in an extensive area over the bilateral striatum and including the anterior cingulate. Talairach coordinates of the voxels with the most significant increases were as follows: x, y, z=−30, 6, −2 with Z=6.68. Additional metabolic increases were observed in the bilateral superior and medial frontal cortex (x, y, z=−6, 64, 28, Z=5.65) and in parts of the right midbrain (x, y, z: 8, −22, −4, Z=4.96) and the left midbrain (x, y, z: −10, −18, −12, Z=4.95). See Table 1a and Figure 1.
Comparison of ethanol influx vs placebo: activations (red ‘hot metal’) of the bilateral striatum, the anterior cingulate, parts of the superior and medial frontal cortex and of the midbrain. Deactivations (blue) of the visual cortex and the left temporo-occipital cortex. See Table 1a and b.
Significant decreases of regional glucose metabolism were found in large areas of the bilateral primary and secondary visual cortex (x, y, z: 20, −98, −2, Z=5.15), the left cuneus/middle and superior occipital cortex (x, y, z: −30, −94, 20, Z=5.84) and the left temporo-occipital cortex (x, y, z: −54, −70, 0, Z=5.09). See Table 1b and Figure 1.
Ethanol Elimination vs Placebo
Subtraction analysis of the comparison ethanol elimination vs placebo yielded similar findings as for the comparison of influx vs placebo showing significant (p<0.05, corrected) increases of regional glucose metabolism in large areas of the bilateral striatum and parts of the anterior cingulate cortex. Talairach coordinates of the voxels with the most significant increases were as follows: x, y, z: −36, 2, −8 with Z=6.82. There was also an increased metabolism in left-sided midbrain areas (x, y, z: −16, −22, −14, Z=5.06). Table 2a.
Regional metabolic decreases were found (very similar to the comparison influx vs placebo) in the bilateral primary and secondary visual cortex and adjacent cuneus (x, y, z: −4, −94, −4, Z=4.93) and in the bilateral parieto-occipital cortex (Brodman area 7) (x, y, z: 2, −76, 42, Z=5.12) (Table 2b).
Ethanol Influx vs Elimination
The categorical comparison of the ethanol kinetic dependent changes revealed significant (p<0.05, corrected) regional metabolic increases during the influx phase in the anterior cingulate (x, y, z: −6, 50, 0, Z=5.41) and the right prefrontal cortex including the Brodman areas 11 and 47 (x, y, z: 44, 30, −12, Z=5.64). See Table 3a and Figure 2a.
(a) Comparison of ethanol influx vs elimination: activations (red ‘hot metal’) of the anterior cingulate (upper row) and of the right prefrontal cortex (lower row). See Table 3a. (b) Comparison of ethanol influx vs elimination: deactivation (blue) of posterior parts of the left superior temporal gyrus including the Wernicke's area. See Table 3b.
Decreases of regional glucose metabolism were found in the left posterior superior temporal gyrus (x, y, z: −44, −26, 4, Z=5.21) comprising parts of the Brodman areas 13, 22, and 41. There was also a significantly reduced metabolism in larger areas of the left temporo-occipital regions including parts of the left parahippocampal gyrus: Brodman areas 11 and 47 (x, y, z: −32, −52, −10, Z=6.05). See Table 3b and Figure 2b.
Blood Alcohol Tests
Blood alcohol concentrations for the influx condition taken at the end of the alcohol infusion (15 min p.i.) ranged from 0.7 to 2.14 ppt (median 1.16 ppt). The blood alcohol concentrations measured 30 min p.i. ranged from 0.44 to 1.34 ppt (median 0.73 ppt). For the elimination condition, blood alcohol concentrations taken 45 min p.i. ranged from 0.44 to 0.77 ppt (median 0.6 ppt).
Neuropsychological Testing
Regarding neuropsychological performance in both subtests (Divided Attention and Flexibility), the subjects demonstrated only during the ethanol influx condition an impairment in terms of longer reaction times (see Table 4). In contrast, there were no differences in reaction times between ethanol elimination condition and placebo. In both subtests, error rates and number of omissions were highest during ethanol influx condition, but differences were not significant.
The task of Divided Attention revealed significant longer reaction times (median) for the ethanol influx condition relative to the elimination condition (repeated-measures ANOVA; post hoc analyses of pairwise differences: p=0.004). Repeated-measures ANOVA only with reactions to the visual stimuli resulted in no significant difference between groups (influx condition relative to elimination condition: p=0.794). Conversely, the reactions to auditory stimuli demonstrated a significantly longer reaction time (median) during the influx condition relative to the elimination condition (repeated-measures ANOVA; post hoc analyses of pairwise differences: p=0.005).
The Flexibility-task resulted in increased reaction time (median) for the influx condition relative to the elimination condition (p=0.005). The difference in reaction time between influx condition and placebo did not reach the statistical threshold (p=0.054).
Correlation Analysis
Since there were significantly longer reaction times in the Divided Attention test exclusively during the influx condition compared to the elimination condition and to placebo, the correlations between the reaction times and the regional cerebral glucose metabolism during the influx phase were investigated in order to detect those cortical areas, which were associated with attentional processes for auditory and visual stimuli, presented under acute ethanol influence.
For auditory stimuli, there was a significantly positive correlation between the median reaction time and the bilateral occipital cortex mainly the secondary visual cortex (right side: x, y, z: 16, −100, −2; Z=3.93; left side x, y, z: −34, −98, −12, Z=3.49) comprising of the Brodman areas 17 and 18 (see Table 5a and Figure 3a). In contrast, the median reaction time for visual stimuli was positively correlated to parts of the left temporal gyrus including auditory areas (x, y, z: −6, −12, −6, Z=3.93) (see Table 5b and Figure 3b). There were no negative correlations above the given statistical threshold.
(a) Ethanol influx condition: Positive correlation (red ‘hot metal’) of the bilateral occipital (secondary visual) cortex activity to the median reaction time for auditory stimuli. See Table 5a. (b) Ethanol influx condition: Positive correlation (red ‘hot metal’) of the left middle and superior temporal (including auditory) cortex activity to the median reaction time for visual stimuli. See Table 5b.
DISCUSSION
The main findings of this study are that acute ethanol administration increases striatal neuronal activity and simultaneously decreases the activity of the primary and secondary visual cortex. The comparison of ethanol influx and ethanol elimination shows an activation in the anterior cingulate and in parts of the right prefrontal association cortex during the influx phase, whereas the activity of perisylvian areas (in particular of the Wernicke area) is decreased. The impaired attentional performance under ethanol influx is correlated to the bilateral occipital and left temporal activity depending on the sensory modality and indicating a reciprocal sensory inhibition for auditory and visual stimuli during acute ethanol impact.
Methodical Considerations
In this study design, we used functional imaging of cerebral neuronal activity by means of 18-FDG PET in order to assess cerebral glucose metabolism as an indicator of neuronal activity. Compared to [O-15]water PET for the measurement of cerebral blood flow with a temporal resolution of 10–30 s (Silbersweig et al, 1993), 18-FDG shows a relatively slower temporal resolution of approximately 20 min, which would indicate that 18-FDG is more appropriate for the investigation of ‘tonic’ stimulation paradigms or pharmacological challenges (Volkow et al, 1990, 1999; Vollenweider et al, 1998; Gouzoulis-Mayfrank et al, 1999; Schreckenberger et al, 1999). Since the psychophysiological changes under acute alcohol influence are rather tonic, they are not expected to be adequately represented by an imaging procedure with very fast time resolution. Therefore, we regarded 18-FDG to be the more suitable tracer for the purpose of this study.
Blood Alcohol Concentrations
All blood alcohol concentrations at the first tests for the influx condition were found to be significantly higher than the calculated theoretical maximum according to the Widmark–Weyrich equation for the ethanol distribution in the human body, using a 0.7 factor and a minimal loss by diffusion of 10%. This demonstrates still undistributed alcohol with a higher concentration in the blood pool compartment. In the influx condition, 30 min after 18-FDG administration the blood alcohol concentrations were either at the calculated maximal values or slightly higher, indicating the last minutes before the alcohol equilibrium. Together, both results prove the cerebral 18-FDG uptake under influx conditions. For the context of the elimination condition, all blood alcohol concentrations should have reached or surpassed the calculated maximal values. This was the case as demonstrated by the samples taken immediately before the 18-FDG administration (45 min p.i.).
Brain Metabolic Ethanol Effects
The most important result of an ethanol induced increased striatal activity might be explained by a stimulation of the ventral striatal ‘reward system’ (Robbins and Everitt, 1996; Koob and Le Moal, 1997). All addictive drugs directly or indirectly influence the dopaminergic projections from the ventral tegmental area (VTA) to the forebrain, and here especially to the amygdala, the ventral pallidum, the prefrontal cortex, and particularly to the nucleus accumbens (NA) (Berke and Hyman, 2000; Wise and Rompre, 1989). The NA plays a crucial role in the reward system of the human brain (Robinson and Berridge, 1993; Koob and Le Moal, 1997; Everitt and Wolf, 2002). Lesions in this brain region abolish the dependence-inducing effects of many drugs of abuse, while stimulation of VTA neurons leads to decreased substance consumption (Di Chiara, 2000). However, the molecular events through which dopamine concentrations are increased in the NA markedly differ between the various drugs of abuse. Stimulants like amphetamine or cocaine increase dopamine release in the NA either directly or by blockade of the dopamine transporter (Reith et al, 1986; Pierce and Kalivas, 1997). Nicotine increases the firing rate of the dopaminergic neurons in the VTA via nicotinic acetylcholine receptors located on the cell bodies of these neurons (Mereu et al, 1987). Opiate receptors of the μ-type are not directly situated on the dopaminergic mesocorticolimbic neurons, but on GABAergic interneurons and GABAergic afferents to the VTA. Stimulation of these receptors leads to inhibition of both tonic and phasic release of GABA, which is secondary followed by disinhibition of dopaminergic VTA neurons (Gysling and Wang, 1983; Dilts and Kalivas, 1989; Pontieri et al, 1996). On the other hand, μ opiate receptors are also found on GABAergic neurons of the NA, which receive the dopaminergic projections from the VTA neurons (Dilts and Kalivas, 1989).
The molecular events that lead to alcohol abuse and dependence are less well defined, which is mainly due to the multiplicity of alcohol actions in the human brain. In general, alcohol inhibits neuronal activity by an amplification of chloride currents and inhibition of glutamate-regulated sodium and calcium currents (Lovinger, 1997; Fadda and Rossetti, 1998). However, alcohol increases the firing rate of dopaminergic VTA neurons and releases dopamine in the NA (Yim et al, 1998; Bunney et al, 2000). These effects can be attributed to inhibition of the GABAergic interneurons of the VTA by alcohol. Since the alcohol-induced dopamine release in the NA can be antagonized by administration of an opiate receptor antagonist (Gonzales and Weiss, 1998), the inhibition of GABAergic VTA interneurons must at least partly be mediated by opioidergic afferents to these neurons. With regard to our study results, the observed ethanol-induced increase of striatal metabolic activity might reflect the disinhibition of dopaminergic VTA neurons (with subsequent dopamine release in the NA), which is most likely due to inhibition of GABAergic interneurons. On the other hand, it is well-known that inhibitory GABA receptors are most densely located in parieto-occipital cortices. Therefore, it is not surprising that the most prominent decreases in metabolic activity are observed in these cortical areas. The fact that these processes occur simultaneously underlines that they share the same biological basis.
The reduced activity of primary and secondary visual cortex, which is also observed by other investigators using oral ethanol administration, might represent the neuronal correlate of the well-described clinical phenomena of a narrowing of the visual field (‘tunnel view’) and the elevated visual perception threshold under acute alcohol impact. It could be shown by vestibular activation studies that a reciprocal inhibitory visual–vestibular interaction might protect visual perception from vestibular mismatches (Brandt et al, 1998). Therefore, further functional imaging studies on the role of alcohol-induced vestibular–visual correlations are mandatory to elucidate this aspect.
Attentional Performance and Neuronal Activity
The well-known fact from clinical observations of more heavily reduced psychophysiological performance during acute alcohol influx compared to a plateau or elimination has a particular psychiatric and forensic relevance. In this context, our finding of an impaired attentional test performance during ethanol influx compared to the ethanol elimination condition and placebo was not surprising but confirms the concept of maximal psychotropic alcohol effects before maximal alcohol uptake is reached. However, the cerebral correlates of this discrepancy between alcohol influx and elimination remained unclear up to now but were not adequately investigated by means of functional brain imaging.
The right prefrontal cortex activation might be associated with an associative thought loosening during acute alcohol influx. Remarkably, stimulation studies indicated activations of the medial frontal cortex and the anterior cingulate as a possible correlate of enhanced attentional processing of alcohol related stimuli (Bush et al, 2000). The finding of reduced activity in perisylvian areas/Wernicke's area during acute influx could be the correlate of an alcohol induced reduced word comprehension but which was not tested in our study design and, therefore, needs further investigation. As our results suggest, a perspective of the further line of research in the field of alcohol intoxication should focus on additional neuropsychological data (eg speech, memory). Neuropsychological testing should include tests of verbal comprehension (eg Verbal Concept Attainment Test, Bornstein and Leason, 1985); verbal fluency (Verbal Fluency Test) Benton and Hamsher, 1989 and tests of verbal memory (Auditory Verbal Learning Test, Rey, 1964; California Verbal Learning Test Delis et al, 1987).
One very interesting result of this study was that the attentional performance under ethanol influx showed reciprocal correlations to the metabolic activity of sensory cortex areas depending on the stimulus modality, which means that higher reduction of occipital (mainly secondary visual) cortical activity is associated with a better test performance for auditory stimuli whereas a higher reduction of left temporal (including auditory areas) cortical activity was correlated with higher attention to visual stimuli. This indicates that suppression of visual cortex activity increases the attentional performance for auditory stimuli and vice versa. Under alcohol induced perceptional changes, a reciprocal inhibitory auditory–visual interaction may provide a successful way to shift the dominant sensorial weight from one modality to the other as a compensatory mechanism. A similar inverse functional correlation in sensory activations could be shown for visual–vestibular stimulations of subjects without pharmacological challenge (Wenzel et al, 1996; Brandt et al, 1998) but was not described for auditory (Benedict et al, 1998; Pedersen et al, 2000, for review see Johnsrude et al, 2002) or visual (Kawashima et al, 1998; Bundesen et al, 2002) attentional processing in pharmacologically unaffected healthy subjects. In this context, the finding of a focal activation of the anterior cingulate during influx vs elimination seems remarkable because the anterior cingulate is considered to have a relevant role for attentional processes in principle and is thought to encode in particular for selective attention (‘selection for attention’) (Demonet et al, 1993; Benedict et al, 1998). Thus, the finding of an increased anterior cingulate neuronal activity despite decreased neuropsychological performance might be the expression of an alcohol induced (insufficient) compensatory additional neuronal recruitment.
CONCLUSION
The present data indicate a close inverse relationship between striatal and occipital (visual) cortical activity under the acute influence of ethanol. Acute ethanol administration in healthy volunteers stimulates those striatal regions, which are considered to have a particular relevance for alcohol craving (‘reward system’). A compensatory reciprocal inhibition of sensory cortex activity seems to be relevant for divided attentional performance during acute alcohol impact.
References
Amberg R, Fuermaier R, Hirt H, Urban R (2002). Regional ethanol absorption of the human brain during the influx phase measured by means of the Magnetic Resonance Spectroscopy (MRS) and Positron Emission Tomography (PET). Blutalkohol 39: 61–71.
Bartenstein P, Weindl A, Spiegel S, Boecker H, Wenzel R, Ceballos-Baumann A et al (1997). Central motor processing in Huntington's disease: a positron emission tomography study. Brain 120: 1553–1567.
Bartlett EJ, Brodie JD, Wolf AP, Christman DR, Laska E, Meissner M (1988). Reproducibility of cerebral glucose metabolic measurements in resting human subjects. J Cereb Blood Flow Metab 8: 502–512.
Benedict RH, Lockwood AH, Shucard JL, Shucard DW, Wack D, Murphy BW (1998). Functional neuroimaging of attention in the auditory modality. Neuroreport 9: 121–126.
Benton AL, Hamsher Kd (1989). Multilingual Aphasia Examination. AJA Associations: Iowa City.
Berke J, Hyman S (2000). Addiction, dopamine, and the molecular mechanisms of memory. Neuron 25: 515–532.
Boecker H, Wills AJ, Ceballos-Baumann A, Samuel M, Thompson PD, Findley LJ et al (1996). The effect of ethanol on alcohol-responsive essential tremor: a positron emission tomography study. Ann Neurol 39: 650–658.
Bornstein RA, Leason M (1985). Effects of localized lesions on the Verbal Concept Attainment Test. J Clin Exp Neuropsychol 7: 421–429.
Brandt T, Bartenstein P, Janek A, Dieterich M (1998). Reciprocal inhibitory visual–vestibular interaction. Visual motion stimulation deactivates the parieto-insular vestibular cortex. Brain 121: 1749–1758.
Bschor F (1952). Beobachtungen über ein funktionales Prinzip bei der psychomotorischen Leistungsminderung in den verschiedenen Phasen der akuten Alkoholintoxikation. Dtsch Z gerichtl Med 41: 273–276.
Bundesen C, Larsen A, Kyllingsbaek S, Paulson OB, Law I (2002). Attentional effects in the visual pathways: a whole-brain PET study. Exp Brain Res 147: 394–406.
Bunney E, Appel S, Brodie M (2000). Cocaine potentiates ethanol-induced excitation of dopaminergic reward neurons in the ventral tegmental area. J Pharmacol Exp Ther 293: 383–389.
Bush G, Luu P, Posner MI (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sciences 4: 215–222.
Delis DC, Kramer JH, Kaplan E, Ober BA (1987). California Verbal Learning Test: Adult Version. The Psychological Corporation: San Antonio, TX.
Demonet JF, Wise R, Frackowiak RSJ (1993). Language functions explored in normal subjects by positron emission tomography: a critical review. Hum Brain Mapping 1: 39–47.
Di Chiara G (2000). Role of dopamine in the behavioural actions of nicotine related to addiction. Eur J Pharmacol 393: 295–314.
Dilts RP, Kalivas PW (1989). Autoradiographic localization of mu-opioid and neurotensin receptors within the mesolimbic dopamine system. Brain Res 488: 311–327.
Everitt BJ, Wolf ME (2002). Psychomotor stimulant addiction: a neural systems perspective. J Neurosci 22: 3312–3320.
Fadda F, Rossetti Z (1998). Chronic ethanol consumption: from neuroadaptation to neurodegeneration. Progr Neurobiol 56: 385–431.
Friston KJ, Ashburner J, Frith CD, Poline JB, Heather JD, Frackowiak RSJ (1995a). Spatial registration and normalization of images. Hum Brain Mapping 3: 165–189.
Friston KJ, Frith CD, Liddle PF, Dolan RJ, Lammertsma AA, Frackowiak RS (1990). The relationship between global and local changes in PET scans. J Cereb Blood Flow Metab 10: 458–466.
Friston KJ, Frith CD, Liddle PF, Frackowiak RS (1991). Comparing functional (PET) images: the assessment of significant change. J Cereb Blood Flow Metab 11: 690–699.
Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ (1995b). Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapping 2: 189–210.
Gonzales RA, Weiss F (1998). Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J Neurosci 18: 10663–10671.
Gouzoulis-Mayfrank E, Schreckenberger M, Sabri O, Arning C, Thelen B, Spitzer M et al (1999). Neurometabolic effects of psilocybin, 3,4-methylenedioxy-ethylamphetamine (MDE) and d-methamphetamine in healthy volunteers. A double-blind, placebo-controlled PET study with [18F]FDG. Neuropsychopharmacology 20: 565–581.
Gysling K, Wang RY (1983). Morphine-induced activation of A10 dopamine neurons in the rat brain. Brain Res 277: 119–127.
Johnsrude IS, Giraud AL, Frackowiak RSJ (2002). Functional imaging of the auditory system: the use of positron emission tomograhy. Audiol Neurootol 7: 251–276.
Kawashima R, Satoh K, Goto R, Inoue K, Itoh M, Fukuda H (1998). The role of the left anterior temporal cortex for visual pattern discrimination – a PET study. Neuroreport 9: 1581–1586.
Koob GF, Le Moal M (1997). Drug abuse: hedonic homeostatic dysregulation. Science 278: 52–58.
Lovinger DM (1997). Alcohols and neurotransmitter gated ion channels: past, present and future. Naunyn-Schmiedeberg's Arch Pharmacol 356: 267–282.
Lumer ED, Friston KJ, Rees G (1998). Neural correlates of perceptual rivalry in the human brain. Science 280: 1930–1934.
Mellanby E (1919). British Medical Research Council, Special Report, Series No. 31.
Mereu G, Yoon K-WP, Boi V, Gessa GL, Nacs L, Westfall TC (1987). Preferential stimulation of ventral tegmental area dopaminergic neurons by nicotine. Eur J Pharmacol 141: 395–399.
Pedersen CB, Mirz F, Ovesen T, Ishizu K, Johannsen P, Madsen S et al (2000). Cortical centres underlying auditory temporal processing in humans: a PET study. Audiology 39: 30–37.
Phelps HE, Huang SC, Hoffmann EJ, Selin C, Sokoloff L, Kuhl DE (1979). Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18) 2-fluoro-2-deoxyglucose: validation of a method. Ann Neurol 6: 371–388.
Pierce RC, Kalivas PW (1997). A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Rev 25: 192–216.
Pontieri FE, Tanda G, Orzi F, Di Chiara G (1996). Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature 382: 255–257.
Reith ME, Meisler BE, Sershen H, Lajtha A (1986). Structural requirements for cocaine congeners to interact with dopamine and serotonin uptake sites in mouse brain and to induce stereotyped behaviour. Biochem Pharmacol 35: 1123–1129.
Reivich M, Alavi A, Wolf AP, Fowler J, Russel J, Arne HC (1985). Glucose metabolic rate kinetic parameter determination in man: the lumped constant for (18-F) fluorodeoxyglucose and (11-C) deoxyglucose. J Cereb Blood Flow Metab 5: 179–192.
Rey A (1964). L' examen clinique en psychologie. Presses Universitaires de France: Paris.
Robbins TW, Everitt BJ (1996). Neurobehavioral mechanisms of reward and motivation. Curr Opin Neurobiol 6: 228–236.
Robinson TE, Berridge KC (1993). The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev 18: 247–291.
Schreckenberger M, Gouzoulis-Mayfrank E, Sabri O, Arning C, Zimny M, Zeggel T et al (1999). ‘Ecstasy’-induced changes of cerebral glucose metabolism and their correlation to acute psychopathology. An 18-FDG PET study. Eur J Nucl Med 26: 1572–1579.
Schwartz JA, Speed NM, Gross MD, Lucey MR, Bazakis AM, Hariharan M et al (1993). Acute effects of alcohol administration on regional cerebral blood flow: the role of acetate. Alcohol Clin Exp Res 17: 1119–1123.
Siebner HR, Limmer C, Peinemann A, Bartenstein P, Drzezga A, Conrad B (2001). Brain correlates of fast and slow handwriting in humans: a PET-performance correlation analysis. Eur J Neurosci 14: 726–736.
Silbersweig DA, Stern E, Frith CD, Cahill C, Schnorr L, Grootoonk S et al (1993). Detection of 30 second cognitive activations in single subjects using a 3-D PET technique. J Cereb Blood Flow Metab 13(Suppl 1): S824.
Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD (1977). The C-14-deoxyglucose method for the measurement of local cerebral glucose utilization: theory procedure and normal values in the conscious and anaesthesized albino rat. J Neurochem 28: 897–916.
Talairach J, Tournoux P (1988). A Co-planar Stereotaxic Atlas of the Human Brain. Stuttgart: Thieme.
Tiihonen J, Kuikka J, Hakola P, Paanila J, Airaksinen J, Eronen M et al (1994). Acute ethanol-induced changes in cerebral blood flow. Am J Psychiatry 151: 1505–1508.
Volkow ND, Hitzemann R, Wolf AP, Logan J, Fowler JS, Christmann D et al (1990). Acute effects of ethanol on regional brain glucose metabolism and transport. Psychiatry Res: Neuroimaging 35: 39–48.
Volkow ND, Wang GJ, Fowler JS, Hitzemann R, Angrist B, Gatley SJ et al (1999). Association of methylphenidate-induced craving with changes in right striato-orbitofrontal metabolism in cocaine abusers: implications in addiction. Am J Psychiatry 156: 19–26.
Vollenweider FX, Maguire RP, Leenders KL, Mathys K, Angst J (1998). Effects of high amphetamine dose on mood and cerebral glucose metabolism in normal volunteers using positron emission tomography (PET). Psychiatry Res 83: 149–162.
Wang GJ, Volkow ND, Franceschi D, Fowler JS, Thanos PK, Scherbaum N et al (2000). Regional brain metabolism during alcohol intoxication. Alcohol Clin Exp Res 24: 822–829.
Wenzel R, Bartenstein P, Dieterich M, Danek A, Weindl A, Minoshima S et al (1996). Deactivation of human visual cortex during involuntary ocular oscillations: a PET activation study. Brain 119: 101–110.
Wise RA, Rompre PP (1989). Brain dopamine and reward. Annu Rev Psychol 40: 191–215.
de Wit H, Metz J, Wagner N, Cooper M (1990). Behavioral and subjective effects of ethanol: relationship to cerebral metabolism using PET. Alcohol Clin Exp Res 14: 482–489.
Worsley KJ, Cao J, Paus T, Petrides M, Evans AC (1998). Applications of random filed theory to functional connectivity. Hum Brain Mapping 6: 364–367.
Worsley KJ, Evans AC, Marrett S, Neelin P (1992). A three- dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab 12: 900–918.
Yim HJ, Schallert T, Randall PK, Gonzales RH (1998). Comparison to local and systemic ethanol effects on extracellular dopamine concentration in rat nucleus accumbens by microdialysis. Alcohol Clin Exp Res 22: 367–374.
Zimmermann P, Fimm B (2002). Testbattery for Attentional Performance (TAP). Version 1.7. Psytest, Freiburg, Germany.
Acknowledgements
This study was supported by Deutsche Forschungsgemeinschaft (DFG): grants Be 454/4-2 and 4-3 and by ‘Bund gegen Alkohol und Drogen im Straßenverkehr, Landessektion Rheinland-Pfalz’. Some of the results of this article form a part of the doctor's thesis of the coauthors Wolfgang Tichy and Andreas Klega at the Medical Faculty of the Johannes Gutenberg-University Mainz, Germany. Furthermore, we thank Mrs Jodi Neverve for her careful review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schreckenberger, M., Amberg, R., Scheurich, A. et al. Acute Alcohol Effects on Neuronal and Attentional Processing: Striatal Reward System and Inhibitory Sensory Interactions under Acute Ethanol Challenge. Neuropsychopharmacol 29, 1527–1537 (2004). https://doi.org/10.1038/sj.npp.1300453
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1300453
Keywords
This article is cited by
-
Intranasal Insulin: a Treatment Strategy for Addiction
Neurotherapeutics (2020)
-
Reduced attentional capture by reward following an acute dose of alcohol
Psychopharmacology (2020)
-
Extracellular cold inducible RNA-binding protein mediates binge alcohol-induced brain hypoactivity and impaired cognition in mice
Molecular Medicine (2019)
-
Effects of the experimental administration of oral estrogen on prefrontal functions in healthy young women
Psychopharmacology (2018)
-
Functional Benefits of (Modest) Alcohol Consumption
Adaptive Human Behavior and Physiology (2017)