Abstract
Oleoylethanolamide (OEA) is a structural analog of the endogenous cannabinoid anandamide, which does not activate cannabinoid receptors. The biosynthesis of OEA in rat small intestine is increased by feeding and reduced by fasting. Moreover, OEA decreases food intake in food-deprived rats via a mechanism that requires intact sensory fibers (Rodríguez de Fonseca, 2001). These results suggest that OEA may contribute to the peripheral regulation of feeding. In the present study, we have investigated the effects of systemic OEA administration (1–20 mg/kg, intraperitoneal) on meal pattern in free-feeding and food-deprived rats. In free-feeding animals, OEA delayed feeding onset in a dose-dependent manner, but had no effect on meal size or postmeal interval. In food-deprived animals, OEA both delayed feeding onset and reduced meal size. The selective effects of OEA in free-feeding rats are strikingly different from those of the serotonergic anorexiant d-fenfluramine (which delayed feeding and reduced meal size) and the intestinal peptide cholecystokinin (which reduced meal size). These results suggest that OEA may participate in the regulation of satiety and may provide a chemical scaffold for the design of novel appetite-suppressing medications.
Similar content being viewed by others
INTRODUCTION
Oleoylethanolamide (OEA) is a natural analog of the endogenous cannabinoid, anandamide (arachidonoylethanolamide) (Bachur and Udenfriend, 1963; Schmid et al, 1996). Like anandamide, OEA is produced by neurons and other cells in a stimulus-dependent manner (Di Marzo et al, 1994; Cadas et al, 1997) and is eliminated through enzymatic hydrolysis (Schmid et al, 1985; Désarnaud et al, 1995, Cravatt et al, 1996), suggesting that it may participate in cell-to-cell signaling processes. Yet, despite its structural and metabolic similarities with anandamide, OEA does not bind to cannabinoid receptors, and its functional roles have long remained elusive.
We have recently reported that OEA, when administered as a drug, dose-dependently decreases eating in food-deprived rats (Rodríguez de Fonseca et al, 2001). This effect may be mediated by peripheral sensory fibers, as it is prevented by treatment with the neurotoxin capsaicin, and is accompanied by discrete activation of brain structures, such as the paraventricular nucleus of the hypothalamus, which are intimately involved in the control of energy balance (Rodríguez de Fonseca et al, 2001). We also have shown that OEA biosynthesis in the small intestine, but not in the brain, is stimulated by feeding and inhibited by fasting, suggesting that this lipid compound may contribute to the intestinal regulation of ingestive behavior (Rodríguez de Fonseca et al, 2001). An opposite role has been proposed for anandamide (Gomez et al, 2002).
The anorexic actions of OEA are remarkably selective. Systemic doses of the compound that strongly inhibit feeding in rats have no effect on a variety of behavioral parameters, including water intake, anxiety, stress-hormone levels, and motor activity (Rodríguez de Fonseca et al, 2001). This selectivity underscores the potential significance of OEA in the physiological regulation of feeding and highlights the interest of defining the mechanism of action of this lipid mediator. With this goal in mind, in the present study we have examined the consequences of OEA administration on meal pattern in food-deprived and free-feeding rats.
MATERIALS AND METHODS
Animals
Adult male Wistar rats (250–300 g) were housed in groups of three in standard Plexiglas cages at a room temperature of 22°C. A 12-h light/dark cycle was set with the light on at 5:30 am. Water and standard chow pellets (Prolab RMH 2500) were available ad libitum. All procedures met the National Institutes of Health guidelines for the care and use of laboratory animals.
Drugs
OEA was synthesized in the laboratory (Giuffrida et al, 2000), dissolved in 70% DMSO/30% sterile saline and administered, 15 min before food presentation, by intraperitoneal (i.p.) injection at doses of 1, 2.5, 5, 10, and 20 mg/kg (in 1 ml/kg vehicle). d-Fenfluramine and cholecystokinin-octapeptide (CCK-8) (Sigma, St Louis, MO) were dissolved in saline containing bovine serum albumin (0.1%, weight/volume). d-Fenfluramine was administered 30 min before food presentation (3 mg/kg, subcutaneously, s.c.), and CCK-8 5 min before food presentation (25 μg/kg, i.p.).
Analysis of Feeding Behavior
Apparatus
Food intake was recorded with an automated system (Scipro Inc., New York, NY), consisting of 24 cages equipped with baskets connected to weight sensors. The baskets contained standard chow pellets and were accessible to the rats through a hole in the wire lid of the cage. Each time food was removed from the basket, the computer recorded the duration of the event, the amount of food retrieved, and the time at which the event occurred. Weight variations were monitored every second and threshold for an eating episode was set at 0.5 g and >1 min.
Procedure
Rats were habituated to the test cages for 3 days prior to trials. Experiments on 24-h food-deprived rats lasted 6 h and were conducted from 10:00 am to 4:00 pm. Experiments with free-feeding rats began at the onset of the dark phase (5:00 pm) and lasted 24 h.
Analysis
Recorded data were analyzed as food ingested per kg body weight per hour, and as cumulative food intake (g/kg body weight) across the test period. A detailed meal analysis was performed adopting a minimum inter-response interval separating two meals of 10 min (Burton et al, 1981). Two categories of feeding parameters were distinguished: ‘first meal parameters’ and ‘average meal parameters’ (Reidelberger et al, 2001). The ‘first meal parameters’ included:
-
Latency of feeding onset (min): the time interval from trial inception to the first eating episode.
-
First meal size (g/kg): amount of food consumed during the first meal.
-
First postmeal interval (min): the time interval between end of the first meal and beginning of the second meal.
-
First satiety ratio [min/(g/kg)]: the ratio between first postmeal interval and first meal size.
The average meal parameters included:
-
Meal size, postmeal interval and satiety ratio: the average of each meal parameter over all meals during the trial period, calculated for each animal.
-
Meal frequency (meals/h): the ratio between total number of meals consumed within the trial period and trial duration.
-
Eating rate ((g/kg)/min): the ratio of the average meal size to average meal duration.
Analysis of OEA Levels in Plasma
Rats were anesthetized with halothane and blood (2 ml) was collected by cardiac puncture at various times after administration of OEA (5 mg/kg, i.p.) or vehicle, using a syringe filled with 1 ml of Krebs-Tris buffer/EDTA (0.1 M). OEA was extracted from plasma and measured by isotope-dilution high-performance liquid chromatography mass spectrometry, as described (Giuffrida et al, 2000).
Statistical Analysis
Cumulative food intake, measured hourly across the test period, was analyzed by two-way analysis of variance (ANOVA), using treatments and time as the two factors. One-way ANOVA, followed by Dunnett's test as post hoc, was used to evaluate the effects of treatments on both the first meal parameters and the average meal parameters. OEA levels in blood samples were analyzed by one-way ANOVA followed by Dunnett's test as post hoc. In each analysis, differences were considered significant if p<0.05.
RESULTS
Effects of OEA in Food-Deprived Rats
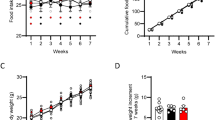
As previously reported (Rodríguez de Fonseca et al, 2001), systemic administration of OEA (20 mg/kg, i.p.) caused a significant inhibition of food intake in rats that had been deprived of food for 24 h (Figure 1). Two-way ANOVA for cumulative food intake gave the following results: F(treatments)=42.55, df=1/60, p<0.001; F(time)=12.67, df=5/60, p<0.0001; F(interaction)=1.72, df=5/60, n.s. After OEA administration, food intake remained significantly lower than baseline for the entire duration of the test, suggesting that OEA-treated animals did not fully compensate for the initial reduction in feeding.
To explore the behavioral basis of this anorexic action, we examined the effects of OEA on meal pattern during a 6-h period after food presentation. As shown in Figure 2, OEA (5 and 20 mg/kg, i.p.) influenced various first-meal parameters in a dose-dependent manner. These parameters included latency of feeding onset (Figure 2a; ANOVA: F=54.59, df=19, p<0.0001), first meal size (Figure 2b; ANOVA: F=26.47, df=19, p<0.0001), and satiety ratio (Figure 2d; ANOVA: F=7.5, df=19, p<0.005). Specifically, OEA increased the latency of feeding onset from a control value of 0.8 to 11.8 min, at 5 mg/kg, and to 42.5 min, at 20 mg/kg (Dunnett's test). Moreover, OEA decreased the first meal size to 54% of control at 5 mg/kg, and to 55% of control at 20 mg/kg (Dunnett's test). By contrast, the compound had no effect on the first postmeal interval at any of the doses tested (Figure 2c). The satiety ratio after the first meal was significantly affected only by the highest dose of OEA (Dunnett's test) (Figure 2d).
Effects of OEA (mg/kg, i.p.) on meal pattern in food-deprived rats. (a) Latency of feeding onset at the beginning of the 6-h trial period. (b–d) First meal parameters. (e–h) Average meal parameters. MS=meal size, PMI=postmeal interval, SR=satiety ratio (variables are defined under Material and Methods). Data represent the mean±SEM of n=5–10; #p<0.05 and ##p<0.01 vs vehicle (Dunnett's test).
OEA also altered various average-meal parameters. These included average-meal size (Figure 2e; ANOVA: F=15.49, df=19, p<0.0001) and postmeal interval (Figure 2f; ANOVA: F=3.95, df=19, p<0.05), both of which were reduced by 5 and 20 mg/kg OEA (Dunnett's test), as well as meal frequency, which was increased by 5 mg/kg OEA (Dunnett's test; Figure 2g; ANOVA: F=5.6, df=19, p<0.05). The parallel decrease in meal size and postmeal interval caused the average satiety ratio to remain unchanged (Figure 2h). The total amount of food intake during the 6-h test was significantly reduced by 20 mg/kg OEA, but not by 5 mg/kg OEA (Dunnett's test) (Figure 3a; ANOVA: F=4.64, df=19, p<0.05). OEA had no effect on the average rate at which rats consumed their meals (Figure 3b).
Effects of OEA in Free-Feeding Rats
Since fasting introduces multiple physiological changes, which may affect the response to anorexiant agents, we next studied the effects of OEA in free-feeding rats. In this experiment, food was removed from test cages only briefly, at the time of OEA injection (15 min before the onset of dark), and intake was continuously monitored for 24 h.
OEA (5–20 mg/kg) produced a dose-dependent inhibition of food intake (Figure 4a). Two-way ANOVA gave the following results: F(treatments)=138.74, df=3/960, p<0.0001; F(time)=171.64, df=23/960, p<0.0001; F(interaction)=0.32, df=69/960, n.s. Significant differences in food intake were observed among treatment groups at 1 h (Figure 4b; ANOVA: F=10.1, df=43, p<0.0001) and 2 h (Figure 4c; ANOVA: F=4.4, df=43, p<0.01) after OEA administration. Analysis of first meal parameters revealed that OEA (1–20 mg/kg) significantly increased the latency of feeding onset at doses of 5, 10, and 20 mg/kg (Dunnett's test) (Figure 5a; ANOVA: F=18.5, df=65, p<0.0001), but had no effect on first meal size, postmeal interval and satiety ratio after the first meal (Figure 5b–d). Analysis of average-meal parameters showed that neither meal size nor postmeal interval was affected by OEA treatment (Figure 5e and f). The highest dose of OEA produced a small, but significant (Dunnett's test) increase in satiety ratio (Figure 5g; ANOVA: F=3.6, df=43, p<0.05). A decrease in meal frequency (Figure 5h; ANOVA: F=10.4, df=43, p<0.0001) was also apparent, and significant after treatment with the doses of 10 and 20 mg/kg (Dunnett's test). As shown in Figure 6a and b, the reduction in meal frequency is accounted for by a decreased number of meals consumed during the dark phase, because of the delay in feeding onset. OEA caused a reduction in total food consumption (ANOVA: F=4.2, df=43, p<0.05), which was significant at doses of 10 and 20 mg/kg (Dunnett's test; Figure 6c). The compound had no effect on eating rate (Figure 6d).
Dose-dependent effects of OEA (mg/kg, i.p.) on cumulative food intake (a) in free-feeding rats during the 24 h test period after OEA administration. The bar represents the light/dark cycle. Effects of OEA on food intake during the first hour (b) and the second hour (c) after dark onset. Data represent the mean±SEM of n=11; #p<0.05 and ##p<0.01 vs vehicle (Dunnett's test).
Effects of OEA (mg/kg, i.p.) on meal pattern in free-feeding rats: (a) latency of feeding onset at the beginning of the 24-h trial period, (b–d) first meal parameters, (e–h) average meal parameters. MS=meal size, PMI=postmeal interval, SR=satiety ratio (variables are defined under Materials and Methods). Data represent the mean±SEM of n=9–15 for latency and n=11 for all other groups of data; #p<0.05 and ##p<0.01 vs vehicle (Dunnett's test).
Plasma OEA Levels After Systemic Administration
Although the animals did not fully compensate for the initial anorexic effects of OEA, such effects were relatively short lived (Figure 4b,c), suggesting that OEA may be rapidly eliminated after systemic administration. To test this possibility, we measured OEA levels in the plasma of free-feeding rats after i.p. injection of a single 5 mg/kg dose of the compound. The time course illustrated in Figure 7 shows that plasma OEA concentrations sharply increased after the injection, returning to baseline levels within 60 min of administration. ANOVA gave the following results: F=10.8, df=35, p<0.0001. A Dunnett's test revealed that OEA levels were significantly different from control at 15 and 30 min, but not at 60 and 120 min after administration. This time course probably reflects a rapid distribution and metabolism of OEA, which is known to be hydrolyzed to oleic acid and ethanolamine by the ubiquitous enzyme fatty acid amide hydrolase (FAAH) (Schmid et al, 1985; Désarnaud et al, 1995; Cravatt et al, 1996).
Effects of d-Fenfluramine and CCK-8 in Free-Feeding Rats
To gain further insight into the mechanism of action of OEA, we compared the anorexic effects of this compound with those of d-fenfluramine and CCK-8 (Burton et al, 1981; Gibbs et al, 1973). d-Fenfluramine (3 mg/kg, s.c.) was administered 30 min before the onset of dark, while CCK-8 (25 μg/kg, i.p.), a short-lived peptide, was administered 5 min before dark. In keeping with its well-known anorexiant actions, d-fenfluramine increased both the latency of feeding onset (ANOVA: F=22.5, df=21, p<0.0001) and first meal size (Figure 8b; Dunnett's test; ANOVA: F=8.89, df=21, p<0.005), whereas CCK-8 had no significant effect on the former (Figure 8a), but decreased the latter (Figure 8b).
DISCUSSION
The main finding of this study is that systemic administration of OEA to free-feeding rats causes a dose-dependent delay in eating onset, which is not accompanied by changes in meal size or postmeal interval. This delay cannot be attributed to motoric inhibition, because it occurs at doses of OEA (5–10 mg/kg) that have no effect on either locomotor activity in the open field test or operant responding for food (Rodríguez de Fonseca et al, 2001). Furthermore, the OEA-induced delay in feeding is unlikely to be because of anxiety or malaise since OEA does not alter performance in the elevated plus-maze test or produce conditioned taste aversion for saccharin (Rodríguez de Fonseca et al, 2001). Therefore, a parsimonious interpretation of our findings, which is in agreement with previous observations (Rodríguez de Fonseca et al, 2001), is that selective doses of OEA inhibit food intake through a direct action on feeding behavior. An additional conclusion suggested by our results is that the effects of OEA may differ mechanistically from those of the serotonergic anorexiant d-fenfluramine, which affects both latency and meal size, and of the intestinal peptide CCK, which only reduces meal size.
A comparison with CCK, whose roles as an intestine-derived satiation factor are well understood (for a review, see Smith, 1999), may help shed light on the possible physiological functions of OEA in feeding. CCK is produced by enteroendocrine I cells of the upper small intestine, from which it is released by the action of fat-containing nutrients. The prandial release of CCK coordinates in a phasic manner multiple alimentary processes, including pancreatic enzyme secretion, bile excretion, and inhibition of food intake. The latter is thought to be, at least in part, mediated through the activation of CCK-A receptors localized to peripheral endings of sensory neurons, and manifests itself behaviorally as a decrease in meal size (Murphy et al, 1992; see Figure 8). OEA also is produced in the intestinal tissue of fed animals and requires intact sensory fibers for its anorexic actions (Rodríguez de Fonseca et al, 2001). However, the effects of this lipid mediator on free-feeding rats differ from those of CCK in that OEA may retard meal initiation rather than accelerate meal termination. Thus, unlike CCK, which contributes in important ways to the process of satiation (the phasic termination of feeding resulting from the act of food ingestion) (Smith, 1999), the primary contribution of OEA to normal feeding may be the regulation of satiety (the tonic state of inhibition over eating).
In contrast with free-feeding rats, food-deprived animals responded to OEA with a combination of increased latency of feeding onset and reduced meal size. This result suggests that OEA may influence meal termination only when the meal size is substantial, as after a period of deprivation. This effect might be due to synergistic interactions of OEA with other gastrointestinal signals originating from the ingestion of large amounts of food, a hypothesis that can be tested experimentally.
In conclusion, our results indicate that OEA selectively delays the normal eating onset in free-feeding rats without affecting meal size and postmeal interval, suggesting that this lipid mediator may participate in the physiological control of satiety.
References
Bachur NR, Udenfriend S (1963). Microsomal synthesis of fatty acid amides. J Biol Chem 241: 1308–1313.
Burton MJ, Cooper SJ, Popplewell DA (1981). The effect of fenfluramine on the microstructure of feeding and drinking in the rat. Br J Pharmac 72: 621–633.
Cadas H, di Tomaso E, Piomelli D (1997). Occurrence and biosynthesis of endogenous cannabinoid precursor, -arachidonoyl phosphatidylethanolamine, in rat brain. J Neurosci 17: 1226–1242.
Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB (1996). Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 384: 83–87.
Désarnaud F, Cadas H, Piomelli D (1995). Anandamide amidohydrolase activity in rat brain microsomes. J Biol Chem 270: 6030–6035.
Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, and Schwartz JC, Piomelli D (1994). Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature 372: 686–691.
Gibbs J, Young RC, Smith GP (1973). Cholecystokinin decreases food intake in rats. J Comp Physiol Psychol 84: 488–495.
Giuffrida A, Rodríguez de Fonseca F, Piomelli D (2000). Quantification of bioactive acylethanolamides in rat plasma by electrospray mass spectrometry. Anal Biochem 280: 87–93.
Gomez R, Navarro M, Ferrer B, Trigo JM, Bilbao A, Del Arco I (2002). A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J Neurosci 22: 9612–9617.
Murphy RB, Smith GP, Schneider LH, Gibbs J (1992). Peripheral factors in the mediation of cholecystokinin-induced satiety as assessed by comparative potencies of cholecystokinin antagonists. Peptides 13: 77–81.
Reidelberger RD, Arnelo U, Granqvist L, Permert J (2001). Comparative effects of amylin and cholecystokinin on food intake and gastric emptying in rats. Am J Physiol Regul Integr Comp Physiol 280: 605–611.
Rodríguez de Fonseca F, Navarro M, Gomez R, Escuredo L, Nava F, Fu J et al (2001). An anorexic lipid mediator regulated by feeding. Nature 414: 209–212.
Schmid HH, Schmid PC, Natarajan V (1996). The N-acylation-phosphodiesterase pathway and cell signaling. Chem Phys Lipids 80: 133–142.
Schmid PC, Zuzarte-Augustin ML, Schmid HH (1985). Properties of rat liver N-acylethanolamine amidohydrolase. J Biol Chem 260: 14145–14149.
Smith GP (1999). Cholecystokinin—The First Twenty-Five Years. In: Bray GA, Ryan DH (eds). Nutrition, genetics, and obesity, volume 9. Louisiana State, University Press: Baton Rouge. pp 227–245.
Acknowledgements
This work was supported by NIDA (grant nos. DA12447, DA12635, DA12413 to DP). The authors thank Dr Fernando Valiño for the synthesis of OEA; Mr Jesse Lo Verme for measuring OEA levels in plasma, and Drs Maurizio Massi, Vincenzo Cuomo, and Fernando Rodríguez de Fonseca for a critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gaetani, S., Oveisi, F. & Piomelli, D. Modulation of Meal Pattern in the Rat by the Anorexic Lipid Mediator Oleoylethanolamide. Neuropsychopharmacol 28, 1311–1316 (2003). https://doi.org/10.1038/sj.npp.1300166
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1300166
Keywords
This article is cited by
-
Plasma endocannabinoids in cocaine dependence and their relation to cerebral metabotropic glutamate receptor 5 density
Translational Psychiatry (2023)
-
Pathophysiological basis and promise of experimental therapies for Gulf War Illness, a chronic neuropsychiatric syndrome in veterans
Psychopharmacology (2023)
-
Structural identification of lysophosphatidylcholines as activating ligands for orphan receptor GPR119
Nature Structural & Molecular Biology (2022)
-
Oleoylethanolamide decreases frustration stress-induced binge-like eating in female rats: a novel potential treatment for binge eating disorder
Neuropsychopharmacology (2020)
-
Cannabinoid CB2 receptors mediate the anxiolytic-like effects of monoacylglycerol lipase inhibition in a rat model of predator-induced fear
Neuropsychopharmacology (2020)