Abstract
Previous studies provide evidence that marijuana (Cannabis sativa) and delta-9-tetrahydrocannabinol (Δ9-THC), the major psychoactive ingredient of marijuana, respectively, are effective in the treatment of tics and behavioral problems in Tourette syndrome (TS). It, therefore, has been speculated that the central cannabinoid receptor system might be involved in TS pathology. However, in healthy marijuana users there is an ongoing debate as to whether the use of cannabis causes acute and/or long-term cognitive deficits. In this randomized double-blind placebo-controlled study, we investigated the effect of a treatment with up to 10 mg Δ9-THC over a 6-week period on neuropsychological performance in 24 patients suffering from TS. During medication and immediately as well as 5–6 weeks after withdrawal of Δ9-THC treatment, no detrimental effect was seen on learning curve, interference, recall and recognition of word lists, immediate visual memory span, and divided attention. Measuring immediate verbal memory span, we even found a trend towards a significant improvement during and after treatment. Results from this study corroborate previous data suggesting that in patients suffering from TS, treatment with Δ9-THC causes neither acute nor long-term cognitive deficits. Larger and longer-duration controlled studies are recommended to provide more information on the adverse effect profile of THC in patients suffering from TS.
Similar content being viewed by others
INTRODUCTION
Gilles de la Tourette syndrome (TS) is a neurobehavioral disorder associated with motor and vocal tics, and a spectrum of behavioral and cognitive features. There is evidence that frontal-subcortical pathways are pathophysiologically involved. Furthermore, the dopaminergic system seems to play a role in TS pathology. Presently, dopamine D2 receptor antagonists (neuroleptics, NL) are the most effective drugs for the treatment of tics (Singer, 2000; Robertson, 2000).
Anecdotal reports (Sandyk and Awerbuch, 1988; Hemming and Yellowlees, 1993; Müller-Vahl et al, 1998,1999) and two controlled studies (Müller-Vahl, 2001; Müller-Vahl et al, 2002) provide evidence that marijuana (Cannabis sativa) and delta-9-tetrahydrocannabinol (Δ9-THC), the major psychoactive ingredient of marijuana, respectively, are effective in the treatment of tics and behavioral problems in TS.
However, human and animal studies suggest that the central cannabinoid receptor (CB1) system is involved in regulating attention, memory, and other cognitive functions (Solowij and Grenyer, 2001). To date, there is a controversial debate as to whether the use of cannabis causes cognitive impairment (Kleiber and Kovar, 1998). In this study we, therefore, investigated the effect of a treatment with up to 10 mg Δ9-THC over a 6-week period on neuropsychological performance. In a randomized double-blind placebo-controlled study in 24 patients suffering from TS, we performed different neuropsychological tests before, during, and after treatment with Δ9-THC.
METHOD
Patients
In this study, 24 adult patients (19 men, five women, mean age=33±11 (SD) years, range, 18–68 years) with TS according to DSM-III R criteria were included. In all, 15 patients were unmedicated and nine were taking medication for the treatment of TS (NL, serotonin-reuptake inhibitors (SRI), clonazepam), which was stable for at least 1 year before entering the study and during the course of the study.
Of these, 17 patients reported that they had never used marijuana before. Four patients reported that they used marijuana occasionally (defined as use one to four times monthly) and three were regular users (defined as use two times or more weekly) during the last year. All patients were asked to stop using marijuana at least 6 weeks before entering the study. To exclude use of cannabis during the last 4–6 weeks in all patients, qualitative urine and quantitative serum tests of Δ9-THC and its metabolites were done at baseline visit.
This study was approved by the local ethic committee, the German Federal Institute for Drugs and Medical Devices (Federal Opium Agency), and the district authority. It has been carried out in accordance with the Declaration of Helsinki. For all patients an insurance was taken out. After complete description of the study to the subjects, written informed consent was obtained.
Treatment
The study was conducted as a prospective, double-blind, placebo-controlled trial. Patients were assigned randomly to receive Δ9-THC (gelatin capsules of 2.5 and 5.0 mg) or identical placebo. Patients assigned to the placebo group received placebo throughout the study. Patients were treated over a period of 6 weeks. The dosage was titrated to a target dosage of 10.0 mg Δ9-THC. Starting at 2.5 mg per day, the dose was increased by increments of 2.5 mg per day every 4 days. The same dosing schedule was used to reduce medication at the end of the treatment period. If a subject could not tolerate the maximum dose, an adjustment could be made by decreasing study medication, until a tolerated dose was achieved. Patients were instructed to take medication once a day in the morning together with breakfast.
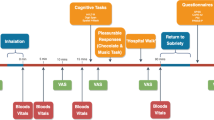
The study consisted of five visits: visit 1, baseline (1 or 2 days before treatment period was started); visit 2, at treatment day 9 (third day at dose 7.5 mg); visit 3, at treatment day 31 (maximum dose); visit 4, at day +1 or +2 (first or second day after study medication was stopped); and visit 5, after 5–6 weeks of study medication withdrawal.
Tests
The following tests were performed to measure cognitive functions.
(1) German version of the auditory verbal learning test (VLMT) (Helmstaedter et al, 2001): This test is suitable to assess immediate verbal memory span, learning curve, interference, word delayed recall, and word recognition. It consists of five presentations with recall of a 15-word list, one presentation of a second 15-word list (interference test), a sixth recall trial of the first word list, and a delayed recognition test after 30 min. A learning curve was obtained over five trials and calculated as the sum of all correct answers. An A- and a B-version were used alternatively. The VLMT was performed at visits 1, 2, 3, 4, and 5.
(2) Benton-visual-retention-test (BVRT) (Benton, 1945): This test measures immediate visual memory span and is sensitive to visual inattention problems. Simple geometric figures have to be drawn from memory after a brief exposure (10 s). The number of errors and correct representations is registered. An A- and a B-version were used alternatively. The BVRT was performed at visits 1, 2, 3, and 4.
(3) Divided attention (TAP) (Zimmermann and Fimm, 1993): In this binary-choice reaction test, subjects have to react differentially to a distinct tone and a specific pattern of white squares on a black screen. Tones and white squares are presented in random order. Reaction time and errors reflect motor speed as well as the decision-making process. This test was performed at visits 1, 2, 3, 4, and 5.
(4) Multiple choice vocabulary test (Mehrfachwahl-Wortschatztest, MWT-B): This test measures verbal intelligence. In a 37-item list, containing five words per item (one correct word and four nonsense words), the correct word has to be identified (Merz et al, 1975). Data were used to correct results obtained from the BVRT for intelligence. This test was performed once at visit 1.
In addition, at each visit blood pressure and pulse were taken. Blood and urine tests of Δ9-THC and its metabolites were performed to exclude additional cannabis use and to control compliance.
Statistical analysis
Data were analyzed using SPSS PC version 10.0 for Windows. Analyses included data only from patients who completed the study and had not to be excluded for any reason. Analyses assessing rates of change involved examinations of change scores (difference between scores at visits 2–5 and baseline visit 1). The significance of differences in THC and placebo group in neuropsychological tests at different examination days was assessed using Mann–Whitney U test. Differences were considered significant if the probability of error was p<0.05. In addition, for the multiple comparisons, the unadjusted p value was compared with the Bonferroni adjusted α of 0.013 (0.05÷4) and 0.017 (0.05÷3), respectively.
Furthermore, we performed repeated measures analysis of variance (ANOVA) to assess differences in neuropsychological tests between both groups. The level of significance was set at the 5% limit.
Influences on neuropsychological tests by other parameters like patients' age and sex, comedication, dosage of Δ9-THC, and prior use of cannabis were tested using repeated measures analysis of covariance (ANCOVA). A value of p<0.05 was used to determine statistical significance.
RESULTS
Four patients dropped out. One patient in the THC group stopped medication at day 4 (first day at dose 5.0 mg) because of the side effects like anxiety and restlessness. Two patients dropped out because of the noncompliance, and in one patient repeated qualitative Δ9-THC urine tests over a 4-week period were positive, although the patient asserted that he had stopped using marijuana weeks before. Therefore, further analyses were performed including a total of 20 patients, nine in the THC group, and 11 in the placebo group. In the THC group, six patients took 10.0 mg Δ9-THC as the maximum dose, two patients 7.5 mg, and one patient 2.5 mg.
Mean absolute values (±SD) at visit 1 for VLMT, BVRT, and TAP are summarized in Table 1,2 and 3. For further analyses, we calculated differences between values measured at visits 2–5 and visit 1. Thus, possible pre-existing group differences before medication was started could be excluded.
Using the VLMT only at visit 4 (1 or 2 days after withdrawal of medication), there was a statistically significant difference between both groups for the parameter memory span (p=0.039) because of more correct answers in the THC group. However, when comparing the results with the Bonferroni adjusted α (0.013) no signifi-cant group differences were seen. ANOVA demonstrated a trend towards a significant difference between both groups for the parameter immediate verbal memory span (p=0.082).
Investigating divided attention (TAP) there were no statistically significant differences between the THC and the placebo group. At visit 5 (5–6 weeks after withdrawal of medication), there was a trend towards a significant difference (p=0.071) because of more correct answers in the THC group. ANOVA demonstrated no significant differences.
Using the BVRT measurements of immediate visual memory span demonstrated no significant differences between the THC and the placebo group. ANOVA also demonstrated no difference.
The MWT-B was used to measure verbal intelligence. Seven TS patients were assessed as moderately intelligent (IQ 91–109), eight as highly intelligent (IQ 110–127), and five as very highly (IQ≥128) intelligent.
Using ANCOVA to investigate the influence on neuro-psychological tests by other parameters, there was an effect for the factor ‘age’ (VLMT-interference, p=0.039, VLMT-recognition, p=0.063; VLMT-recall, p=0.073; BVRT-n errors, p=0.074). No effect was seen for the factors such as sex, comedication, dosage of Δ9-THC, and prior use of cannabis.
No serious adverse reactions occurred. Pulse and blood pressure did not change significantly during Δ9-THC treatment. Urine and serum analyses of Δ9-THC and its metabolites demonstrated patients' compliance and that no patient in the placebo group used marijuana additionally.
DISCUSSION
Clinical trials provide evidence that Δ9-THC is effective in the treatment of motor and vocal tics and associated behavioral problems such as obsessive–compulsive behavior in TS (Müller-Vahl, 2001; Müller-Vahl et al, 2002). Previous studies in healthy marijuana users, however, suggested that cannabinoids may cause acute and/or long-term effects on cognition (Kleiber and Kovar, 1998).
Results from this study demonstrated no significant deterioration in cognitive functions in TS patients during a 6-week Δ9-THC treatment. Quite the reverse, using the VLMT we found a trend towards a significant difference with more correct answers in the THC group concerning the parameter ‘immediate memory span’. Other parameters measured by the VLMT such as learning, interference, recall, and recognition remained unchanged during and after the Δ9-THC treatment. Immediate visual memory measured by the BVRT as well as divided attention measured by the TAP did not demonstrate significant differences between the THC and the placebo group. Withdrawal of Δ9-THC medication as well did not result in a worsening of neuropsychological performances. As expected, further analyses demonstrated an influence on neuropsychological performance by patients' age.
Therefore, our data are in agreement with a pilot study in 12 TS patients demonstrating no detrimental effect on verbal and visual memory, sustained and divided attention, reaction time, intelligence, and vigilance after a single-dose treatment with up to 10 mg Δ9-THC (Müller-Vahl et al, 2001). From anecdotal reports, it is even suggested that in TS patients smoked marijuana might improve attention (Müller-Vahl et al, 1998,1999; Sandyk and Awerbuch, 1988).
Previous investigations on cognitive functions in healthy marijuana users are inconsistent. In acute intoxicated persons, an impairment of reaction time, short-term memory, recall, recognition, and attention has been observed (Hall et al, 1994; Hampson and Deadwyler, 1999; Leweke et al, 1998). Furthermore, alterations in associative and perceptual processes have been described (Emrich et al, 1997; Schneider et al, 1998; Block et al, 1992). However, in experienced marijuana users only minimal detrimental effects of acute smoked marijuana on complex cognitive task performance were seen (Hart et al, 2001). Investigating long-term effects of cannabis, available studies suggest that cognitive functions are not grossly impaired. There is evidence that long-term use leads to a more subtle and selective impairment in higher cognitive functions such as selective and focused attention, visual and verbal memory, and the organization and integration of complex information (Solowij, 1998; Pope and Yurgelun-Todd, 1996; Fletcher et al, 1996). In addition, there is evidence that both duration and frequency of use influence cognitive impairments (Solowij and Grenyer, 2001). However, there is ongoing debate as to whether these impairments can be reversed by abstinence and how important they are for everyday functioning (Hall and Solowij, 1997). Furthermore, it is unclear whether deficits detected are because of the accumulation of cannabinoids, the withdrawal from the drug, or a more lasting alteration of brain function caused by a frank neurotoxic effect (Pope and Yurgelun-Todd, 1996). In a recent study (Pope et al, 2001), a neuropsychological test battery was administered to 108 current and former heavy cannabis users and 72 control subjects. At least 7 days after heavy cannabis use was stopped, cognitive deficits on memory of word lists were detectable. No significant differences could be observed 4 weeks after use, suggesting that cannabis-induced cognitive deficits were reversible and not related to cumulative lifetime use.
Since previous studies in TS (Müller-Vahl, 2001, Müller-Vahl et al, 2002) have demonstrated a significant reduction of tics after treatment with Δ9-THC, it can be speculated that the central CB1 receptor system might be involved in the pathology of the disease. The endogenous CB1 receptor system is thought to play a role in both motor control and memory, emotion, and other cognitive functions (Hall and Solowij, 1998). Central cannabinoid CB1 receptors have been found to be located at high concentrations in the output nuclei of the basal ganglia, in forebrain areas associated with higher cognitive functions, in the molecular layers of the cerebellum, hippocampal dentate gyrus, and other parts of the hippocampal formation (Herkenham et al, 1990). Assuming an involvement of the CB1 receptor system in TS pathophysiology, it can be speculated that unchanged or even improved cognitive functions after Δ9-THC treatment are because of a pre-existing dysfunction in the cannabinoid receptor system, and that the influence of cannabinoids on cognitive processes might be different compared to healthy users.
On the other hand, it can be speculated that in this study no detrimental effect on cognitive functions was seen because patients were treated with pure Δ9-THC. In contrast, in the large majority of studies in healthy subjects, the effect of marijuana on cognitive functions was investigated. To date, it is unclear whether cognitive deficits detected in healthy marijuana users are because of Δ9-THC, other cannabinoids or noncannabinoid ingredients of C. sativa (Solowij and Grenyer, 2001).
Some limitations of our study should be considered. First, the sample size was relatively small. Second, patients were treated only over a period of 6 weeks. Therefore, no statement can be made whether a longer-term treatment would cause cognitive deficits. To exclude completely a detrimental effect of THC on neuropsychological performance, a larger and longer-duration controlled study is needed. Third, we used a small test battery consisting of three neuropsychological tests. Therefore, it cannot entirely be excluded that cognitive deficits would be detected when using a larger test battery. However, in their recent study, Pope et al (2001) used a test battery consisting of 10 tests and the only significant difference between heavy cannabis users and normal controls they found was seen in memory of word lists on the Buschke selective reminding test (BSRT) (Buschke, 1973). Using the VLMT in this study, no detrimental effect of Δ9-THC on recall of word lists could be measured.
In conclusion, our data are in agreement with anecdotal reports and a pilot study suggesting that Δ9-THC treatment in patients suffering from TS has no detrimental effect on neuropsychological performance. We hypothesize that the effects of Δ9-THC on cognition in TS patients might be different from those in healthy marijuana users because of the pathology of the disease. Since there is evidence that tics can be improved by Δ9-THC, an involvement of the central CB1 receptor system in TS pathology has been suggested. However, larger and longer-duration controlled studies are recommended to provide more information on the adverse effect profile of THC in patients suffering from TS.
References
Benton AL (1945). A visual retention test for clinical use. Arch Neurol Psychol 59: 273–291.
Block RI, Farinpour R, Braverman K (1992). Acute effects of marijuana on cognition: relationships to chronic effects and smoking techniques. Pharmacol Biochem Behav 43: 907–917.
Buschke H (1973). Selective reminding of analyses of memory and learning. J Verbal Learning Verbal Behav 12: 543–550.
Emrich HM, Leweke FM, Schneider U (1997). Towards a cannabinoid hypothesis of schizophrenia: cognitive impairments due to dysregulation of the endogenous cannabinoid system. Pharmacol Biochem Behav 56: 803–807.
Fletcher JM, Page JB, Francis DJ, Copeland K, Naus MJ, Davis CM et al (1996). Cognitive correlates of long-term cannabis use in Costa Rican men. Arch Gen Psychiatry 53: 1051–1057.
Hall W, Solowij N (1997). Long-term cannabis use and mental health. Br J Psychiatry 171: 107–108.
Hall W, Solowij N (1998). Adverse effects of cannabis. Lancet 14: 1611–1616.
Hall W, Solowij N, Lemon J (1994). The Health and Psychological Consequences of Cannabis Use. National Drug Strategy Monograph Series no 25. Australian Government Publishing Service: Canberra.
Hampson RE, Deadwyler SA (1999). Cannabinoids, hippocampal function and memory. Life Sci 65: 715–723.
Hart CL, van Gorp W, Haney M, Foltin RW, Fischman MW (2001). Effects of acute smoked marijuana on complex cognitive performance. Neuropsychopharmacology 25: 757–765.
Helmstaedter C, Lendt M, Lux S (2001). VLMT—verbaler Lern- und Merkfähigkeitstest. Hogrefe Verlag: Beltz Test GmbH.
Hemming M, Yellowlees PM (1993). Effective treatment of Tourette's syndrome with marijuana. J Psychopharmacol 7: 389–391.
Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR et al (1990). Cannabinoid receptor localization in brain. Proc Natl Acad Sci USA 87: 1932–1936.
Kleiber D, Kovar K-A (1998). Psychische und soziale Konsequenzen des Cannabiskonsums. In: Kleiber D, Kovar K-A (eds). Auswirkungen des Cannabiskonsums: eine Expertise zu pharmakologischen und psychosozialen Konsequenzen. Wissenschaft-liche Verlagsgesellschaft mbH: Stuttgart. pp 88–225.
Leweke M, Kampmann C, Radwan M, Dietrich DE, Johannes S, Emrich HM et al (1998). The effects of tetrahydrocannabinol on the recognition of emotionally charged words: an analysis using event-related brain potentials. Neuropsychobiology 37: 104–111.
Merz J, Lehrl S, Galster JV, Erzigkeit H (1975). MWT-B—ein Intelligenzkurztest. Psychiatr Neurol Med Psychol 27: 423–428.
Müller-Vahl KR (2001). Cannabinoide bei Bewegungsstörungen. Nervenheilkunde 20 (Suppl 3): S72–S73.
Müller-Vahl KR, Koblenz A, Jöbges M, Kolbe H, Emrich HM, Schneider U (2001). Influence of delta-9-tetrahydrocannabinol (Δ9-THC) treatment of Tourette-syndrome on neuropsychological testing. Pharmacopsychiatry 34: 19–24.
Müller-Vahl KR, Kolbe H, Schneider U, Emrich HM (1998). Cannabinoids: possible role in pathophysiology of Gilles de la Tourette-syndrome. Acta Psychiat Scand 98: 502–506.
Müller-Vahl KR, Schneider U, Koblenz A, Jöbges M, Kolbe H, Daldrup T et al (2002). Treatment of Tourette-syndrome with Δ9-tetrahydrocannabinol (THC). Pharmacopsychiatry 35: 57–61.
Müller-Vahl KR, Schneider U, Kolbe H, Emrich HM (1999). Treatment of Tourette-syndrome with delta-9-tetrahydrocannabinol. Am J Psychiatry 156: 395.
Pope Jr HG, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D (2001). Neuropsychological performance in long-term cannabis users. Arch Gen Psychiatry 58: 909–915.
Pope Jr HG, Yurgelun-Todd D (1996). The residual cognitive effects of heavy marijuana use in college students. JAMA 21: 521–527.
Robertson MM (2000). Tourette syndrome, associated conditions and the complexities of treatment. Brain 123: 425–462.
Sandyk R, Awerbuch G (1988). Marijuana and Tourette's syndrome. J Clin Psychopharmacol 8: 333–335.
Schneider U, Leweke FM, Mueller-Vahl KR, Emrich HM (1998). Cannabinoid/anandamide system and schizophrenia: is there evidence for association? Pharmacopsychiatry 31 (Suppl 2): 110–113.
Singer HS (2000). Current issues in Tourette syndrome. Mov Disord 15: 1051–1063.
Solowij N (1998). Cannabis and Cognitive Functioning. Cambridge University Press: Cambridge.
Solowij N, Grenyer BFS (2001). Langzeiteffekte auf Psyche und Kognition. In: Grotenhermen F (ed). Cannabis und Cannabinoide. Pharmakologie, Toxikologie und therapeutisches Potential. Verlag Hans Huber: Bern. pp 325–338.
Zimmermann P, Fimm B (1992). Testbatterie zur Aufmerksam keits prufung (TAP). Psy text: Wörselew.
Acknowledgements
This study was supported by a grant of the Medical School of Hannover. We thank Dr Wiese for her help with the statistical analysis. Δ9-THC was kindly donated by UNIMED Pharmaceuticals, Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Müller-Vahl, K., Prevedel, H., Theloe, K. et al. Treatment of Tourette Syndrome with Delta-9-Tetrahydrocannabinol (Δ9-THC): No Influence on Neuropsychological Performance. Neuropsychopharmacol 28, 384–388 (2003). https://doi.org/10.1038/sj.npp.1300047
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1300047
Keywords
This article is cited by
-
Medical cannabinoids: a pharmacology-based systematic review and meta-analysis for all relevant medical indications
BMC Medicine (2022)
-
The effect of medical cannabis on cognitive functions: a systematic review
Systematic Reviews (2022)
-
Randomized controlled trials on the use of cannabis-based medicines in movement disorders: a systematic review
Journal of Neural Transmission (2022)
-
The why behind the high: determinants of neurocognition during acute cannabis exposure
Nature Reviews Neuroscience (2021)
-
Update on the Treatment of Tics in Tourette Syndrome and Other Chronic Tic Disorders
Current Treatment Options in Neurology (2020)