Abstract
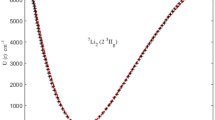
THE law of interaction of two colliding molecules follows the curve shown in Fig. 1, where the mutual potential energy U of the molecules is plotted against the distance r between their nuclei. In the case of molecules with saturated bonds, U is determined by the polarisation forces and repulsive forces, the nature of which we do not propose to discuss here. When the chemical bonds are unsaturated, the ‘chemical forces’ (Austauscheffekt) also must be taken into account. In both cases the energy minimum (U0) corresponds to a definite stationary state of the associated molecule. Such a twin will appear if the molecules lose during the collision a certain amount of their energy, for example by means of a triple collision, so that the total energy attains a negative value. The dissociation energy W of this twin is equal to −U0 (Fig. 1).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
BRESSLER, S., KONDRATJEW, V. The Heat of Dissociation of the Molecule O4 and Sutherland's Constant for Oxygen. Nature 125, 164–165 (1930). https://doi.org/10.1038/125164a0
Issue Date:
DOI: https://doi.org/10.1038/125164a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.