Abstract
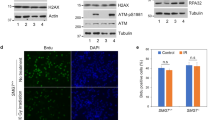
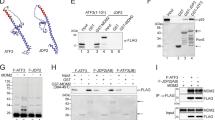
In response to stress, p53 is accumulated and activated to induce appropriate growth inhibitory responses. This requires the release of p53 from the constraints of its negative regulators Mdm2 and Mdm4. A key event in this dissociation is the phosphorylation of p53 at threonine residue (Thr18) within the Mdm2/4-binding domain. Casein kinase 1 (CK1) plays a major role in this phosphorylation. The promyelocytic leukemia protein (PML) regulates certain modifications of p53 in response to DNA damage. Here, we investigated the role of PML in the regulation of Thr18 phosphorylation. We found that PML enhances Thr18 phosphorylation of endogenous p53 in response to stress. On DNA damage, CK1 accumulates in the cell, with a proportion concentrated in the nucleus together with p53 and PML. Furthermore, CK1 interacts with endogenous p53 and PML, and this interaction is enhanced by genotoxic stress. Inhibition of CK1 impairs the protection of p53 by PML from Mdm2-mediated degradation. Our findings support a role for PML in the regulation of p53 by CK1. We propose that following DNA damage, PML facilitates Thr18 phosphorylation by recruiting p53 and CK1 into PML nuclear bodies, thereby protecting p53 from inhibition by Mdm2, leading to p53 activation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Behrend L, Milne DM, Stöter M, Deppert W, Campbell LE, Meek DW et al. (2000). IC261, a specific inhibitor of the protein kinases casein kinase 1-delta and -epsilon, triggers the mitotic checkpoint and induces p53-dependent postmitotic effects. Oncogene 19: 5303–5313.
Craig AL, Burch L, Vojtesek B, Mikutowska J, Thompson A, Hupp TR . (1999). Novel phosphorylation sites of human tumour suppressor protein p53 at Ser20 and Thr18 that disrupt the binding of mdm2 (mouse double minute 2) protein are modified in human cancers. Biochem J 342 (Pt 1): 133–141.
de Stanchina E, Querido E, Narita M, Davuluri RV, Pandolfi PP, Ferbeyre G et al. (2004). PML is a direct p53 target that modulates p53 effector functions. Mol Cell 13: 523–535.
Dumaz N, Milne DM, Meek DW . (1999). Protein kinase CK1 is a p53-threonine 18 kinase which requires prior phosphorylation of serine 15. FEBS Lett 463: 312–316.
Gurrieri C, Capodieci P, Bernardi R, Scaglioni PP, Nafa K, Rush LJ et al. (2004). Loss of the tumor suppressor PML in human cancers of multiple histologic origins. J Natl Cancer Inst 96: 269–279.
Hoekstra MF, Dhillon N, Carmel G, DeMaggio AJ, Lindberg RA, Hunter T et al. (1994). Budding and fission yeast casein kinase I isoforms have dual-specificity protein kinase activity. Mol Biol Cell 5: 877–886.
Iwakuma T, Lozano G . (2003). MDM2, an introduction. Mol Cancer Res 1: 993–1000.
Knippschild U, Gocht A, Wolff S, Huber N, Löhler J, Stöter M . (2005). The casein kinase 1 family: participation in multiple cellular processes in eukaryotes. Cell Signal 17: 675–689.
Knippschild U, Milne DM, Campbell LE, DeMaggio AJ, Christenson E, Hoekstra MF et al. (1997). p53 is phosphorylated in vitro and in vivo by the delta and epsilon isoforms of casein kinase 1 and enhances the level of casein kinase 1 delta in response to topoisomerase-directed drugs. Oncogene 15: 1727–1736.
Lai Z, Auger KR, Manubay CM, Copeland RA . (2000). Thermodynamics of p53 binding to hdm2 (1–126): effects of phosphorylation and p53 peptide length. Arch Biochem Biophys 381: 278–284.
Langley E, Pearson M, Faretta M, Bauer UM, Frye RA, Minucci S et al. (2002). Human SIR2 deacetylates p53 and antagonizes PML/p53-induced cellular senescence. EMBO J 21: 2383–2396.
Lavin MF, Gueven N . (2006). The complexity of p53 stabilization and activation. Cell Death Differ 13: 941–950.
Louria-Hayon I, Grossman T, Sionov RV, Alsheich O, Pandolfi PP, Haupt Y . (2003). The promyelocytic leukemia protein protects p53 from Mdm2-mediated inhibition and degradation. J Biol Chem 278: 33134–33141.
Marine JC, Dyer MA, Jochemsen AG . (2007). MDMX: from bench to bedside. J Cell Sci 120: 371–378.
Maritzen T, Lohler J, Deppert W, Knippschild U . (2003). Casein kinase I delta (CKIdelta) is involved in lymphocyte physiology. Eur J Cell Biol 82: 369–378.
Mashhoon N, DeMaggio AJ, Tereshko V, Bergmeier SC, Egli M, Hoekstra MF et al. (2000). Crystal structure of a conformation-selective casein kinase-1 inhibitor. J Biol Chem 275: 20052–20060.
Meek DW . (2004). The p53 response to DNA damage. DNA Repair (Amst) 3: 1049–1056.
Muller S, Miller Jr WH, Dejean A . (1998). Trivalent antimonials induce degradation of the PML-RAR oncoprotein and reorganization of the promyelocytic leukemia nuclear bodies in acute promyelocytic leukemia NB4 cells. Blood 92: 4308–4316.
Rizzo MG, Zepparoni A, Cristofanelli B, Scardigli R, Crescenzi M, Blandino G et al. (1998). Wt-p53 action in human leukaemia cell lines corresponding to different stages of differentiation. Br J Cancer 77: 1429–1438.
Saito S, Yamaguchi H, Higashimoto Y, Chao C, Xu Y, Fornace Jr AJ et al. (2003). Phosphorylation site interdependence of human p53 post-translational modifications in response to stress. J Biol Chem 278: 37536–37544.
Sakaguchi K, Saito S, Higashimoto Y, Roy S, Anderson CW, Appella E . (2000). Damage-mediated phosphorylation of human p53 threonine 18 through a cascade mediated by a casein 1-like kinase. Effect on Mdm2 binding. J Biol Chem 275: 9278–9283.
Salomoni P, Pandolfi PP . (2002). p53 De-ubiquitination: at the edge between life and death. Nat Cell Biol 4: E152–E153.
Schon O, Friedler A, Bycroft M, Freund SM, Fersht AR . (2002). Molecular mechanism of the interaction between MDM2 and p53. J Mol Biol 323: 491–501.
Sionov RV, Coen S, Goldberg Z, Berger M, Bercovich B, Ben-Neriah Y et al. (2001). c-Abl regulates p53 levels under normal and stress conditions by preventing its nuclear export and ubiquitination. Mol Cell Biol 21: 5869–5878.
Soubeyrand S, Schild-Poulter C, Haché RJ . (2004). Structured DNA promotes phosphorylation of p53 by DNA-dependent protein kinase at serine 9 and threonine 18. Eur J Biochem 271: 3776–3784.
Trotman LC, Alimonti A, Scaglioni PP, Koutcher JA, Cordon-Cardo C, Pandolfi PP . (2006). Identification of a tumour suppressor network opposing nuclear Akt function. Nature 441: 523–527.
Wang ZG, Delva L, Gaboli M, Rivi R, Giorgio M, Cordon-Cardo C et al. (1998). Role of PML in cell growth and the retinoic acid pathway. Science 279: 1547–1551.
Wolff S, Stoter M, Giamas G, Piesche M, Henne-Bruns D, Banting G et al. (2006). Casein kinase 1 delta (CK1delta) interacts with the SNARE associated protein snapin. FEBS Lett 580: 6477–6484.
Zhu J, Koken MH, Quignon F, Chelbi-Alix MK, Degos L, Wang ZY et al. (1997). Arsenic-induced PML targeting onto nuclear bodies: implications for the treatment of acute promyelocytic leukemia. Proc Natl Acad Sci USA 94: 3978–3983.
Zimber A, Nguyen QD, Gespach C . (2004). Nuclear bodies and compartments: functional roles and cellular signaling in health and disease. Cell Signal 16: 1085–1104.
Acknowledgements
We thank Stefan Muller and PP Pandolfi for their generous gifts of expression plasmids, Anthony DeMaggio and Dan Eilat for antibodies. PML-knockout MEFs were a kind gift from PP Pandolfi. This work was supported by grants from the Association for International Cancer Research, the German-Israeli Foundation for Scientific Research and Development, the Israel Science Foundation (Grant No. 1341/05), in part, by the Intramural Research Program of the NIH, NCI, CCR and by EC FP6 funding of the European Commission (contract 503576). This publication reflects only the authors' views. The European Commission is not liable for any use that may be made of the information here.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Supplementary information
Rights and permissions
About this article
Cite this article
Alsheich-Bartok, O., Haupt, S., Alkalay-Snir, I. et al. PML enhances the regulation of p53 by CK1 in response to DNA damage. Oncogene 27, 3653–3661 (2008). https://doi.org/10.1038/sj.onc.1211036
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1211036
Keywords
This article is cited by
-
Liquid-like droplet formation by tumor suppressor p53 induced by multivalent electrostatic interactions between two disordered domains
Scientific Reports (2020)
-
TRIM52 plays an oncogenic role in ovarian cancer associated with NF-kB pathway
Cell Death & Disease (2018)
-
TRIMming p53’s anticancer activity
Oncogene (2016)
-
The role of PML in the control of apoptotic cell fate: a new key player at ER–mitochondria sites
Cell Death & Differentiation (2011)
-
Seed-expressed casein kinase I acts as a positive regulator of the SeFAD2 promoter via phosphorylation of the SebHLH transcription factor
Plant Molecular Biology (2010)