Abstract
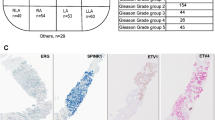
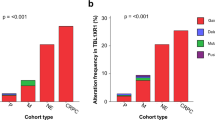
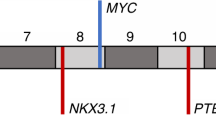
An ERG gene ‘break-apart’ fluorescence in situ hybridization (FISH) assay has been used to screen whole-mount prostatectomy specimens for rearrangements at the ERG locus. In cancers containing ERG alterations the observed pattern of changes was often complex. Different categories of ERG gene alteration were found either together in a single cancerous region or within separate foci of cancer in the same prostate slice. In some cases the juxtaposition of particular patterns of ERG alterations suggested possible mechanisms of tumour progression. Prostates harbouring ERG alterations commonly also contained cancer that lacked rearrangements of the ERG gene. A single trans-urethral resection of the prostate specimen examined harboured both ERG and ETV1 gene rearrangements demonstrating that the observed complexity may, at least in part, be explained by multiple ETS gene alterations arising independently in a single prostate. In a search for possible precursor lesions clonal ERG rearrangements were found both in high grade prostatic intraepithelial neoplasia (PIN) and in atypical in situ epithelial lesions consistent with the diagnosis of low grade PIN. Our observations support the view that ERG gene alterations represent an initiating event that promotes clonal expansion initially to form regions of epithelial atypia. The complex patterns of ERG alteration found in prostatectomy specimens have important implications for the design of experiments investigating the clinical significance and mechanism of development of individual prostate cancers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Aihara M, Wheeler TM, Ohori M, Scardino PT . (1994). Heterogeneity of prostate cancer in radical prostatectomy specimens. Urology 43: 60–66.
Arora R, Koch MO, Eble JN, Ulbright TM, Li L, Cheng L . (2004). Heterogeneity of Gleason grade in multifocal adenocarcinoma of the prostate. Cancer 100: 2362–2366.
Attard G, Clark J, Ambroisine L, Fisher G, Kovacs G, Flohr P et al. (2007). Duplication of the fusion of TMPRSS2 to ERG sequences identifies fatal human prostate cancer. Oncogene; e-pub ahead of print: 16 July 2007.
Bostwick DG, Shan A, Qian J, Darson M, Maihle NJ, Jenkins RB et al. (1998). Independent origin of multiple foci of prostatic intraepithelial neoplasia: comparison with matched foci of prostate carcinoma. Cancer 83: 1995–2002.
Brawer MK, Bigler SA, Sohlberg OE, Nagle RB, Lange PH . (1991). Significance of prostatic intraepithelial neoplasia on prostate needle biopsy. Urology 38: 103–107.
Cerveira N, Ribeiro FR, Peixoto A, Costa V, Henrique R, Jeronimo C et al. (2006). TMPRSS2–ERG gene fusion causing erg overexpression precedes chromosome copy number changes in prostate carcinomas and paired HGPIN lesions. Neoplasia 8: 826–832.
Chen ME, Johnston DA, Tang K, Babaian RJ, Troncoso P . (2000). Detailed mapping of prostate carcinoma foci: biopsy strategy implications. Cancer 89: 1800–1809.
Cheng L, Song SY, Pretlow TG, Abdul-Karim FW, Kung HJ, Dawson DV et al. (1998). Evidence of independent origin of multiple tumors from patients with prostate cancer. J Natl Cancer Inst 90: 233–237.
Chun FK, Briganti A, Jeldres C, Erbersdobler A, Schlomm T, Steuber T et al. (2007). Zonal origin of localized prostate cancer does not affect the rate of biochemical recurrence after radical prostatectomy. Eur Urol 51: 949–955.
Clark J, Merson S, Jhavar S, Flohr P, Edwards S, Foster CS et al. (2006). Diversity of TMPRSS2–ERG fusion transcripts in the human prostate. Oncogene 26: 2667–2673.
Cuzick J, Fisher C, Kattan MW, Berney D, Oliver T, Foster CS et al. (2006). Long-term outcome among men with conservatively treated localised prostate cancer. Br J Cancer 95: 1186–1194.
Demichelis F, Fall K, Perner S, Andren O, Schmidt F, Setlur SR et al. (2007). TMPRSS2:ERG gene fusion associated with lethal prostate cancer in a watchful waiting cohort. Oncogene 26: 5692.
Djavan B, Susani M, Bursa B, Basharkhah A, Simak R, Marberger M . (1999). Predictability and significance of multifocal prostate cancer in the radical prostatectomy specimen. Tech Urol 5: 139–142.
Egevad L, Allsbrook WC, Epstein JI . (2006). Current practice of diagnosis and reporting of prostatic intraepithelial neoplasia and glandular atypia among genitourinary pathologists. Mod Pathol 19: 180–185.
Epstein JI, Grignon DJ, Humphrey PA, McNeal JE, Sesterhenn IA, Troncoso P et al. (1995). Interobserver reproducibility in the diagnosis of prostatic intraepithelial neoplasia. Am J Surg Pathol 19: 873–886.
Foster CS . (2000). Pathology of benign prostatic hyperplasia. Prostate Suppl 9: 4–14.
Foster CS, Bostwick DG, Bonkhoff H, Damber JE, van der KT, Montironi R et al. (2000). Cellular and molecular pathology of prostate cancer precursors. Scand J Urol Nephrol Suppl, 19–43.
Foster CS, Falconer A, Dodson AR, Norman AR, Dennis N, Fletcher A et al. (2004). Transcription factor E2F3 overexpressed in prostate cancer independently predicts clinical outcome. Oncogene 23: 5871–5979.
Greene DR, Fitzpatrick JM, Scardino PT . (1995). Anatomy of the prostate and distribution of early prostate cancer. Semin Surg Oncol 11: 9–22.
Grignon DJ, Sakr WA . (1994). Zonal origin of prostatic adenocarcinoma: are there biologic differences between transition zone and peripheral zone adenocarcinomas of the prostate gland? J Cell Biochem Suppl 19: 267–269.
Hermans KG, van Marion R, van Dekken H, Jenster G, van Weerden WM, Trapman J . (2006). TMPRSS2:ERG fusion by translocation or interstitial deletion is highly relevant in androgen-dependent prostate cancer, but is bypassed in late-stage androgen receptor-negative prostate cancer. Cancer Res 66: 10658–10663.
Iljin K, Wolf M, Edgren H, Gupta S, Kilpinen S, Skotheim RI et al. (2006). TMPRSS2 fusions with oncogenic ETS factors in prostate cancer involve unbalanced genomic rearrangements and are associated with HDAC1 and epigenetic reprogramming. Cancer Res 66: 10242–10246.
Jenkins RB, Qian J, Lieber MM, Bostwick DG . (1997). Detection of C-Myc oncogene amplification and chromosomal anomalies in metastatic prostatic carcinoma by fluorescence in situ hybridization. Cancer Res 57: 524–531.
Jhavar SG, Fisher C, Jackson A, Reinsberg SA, Dennis N, Falconer A et al. (2005). Processing of radical prostatectomy specimens for correlation of data from histopathological, molecular biological, and radiological studies: a new whole organ technique. J Clin Pathol 58: 504–508.
Konishi N, Hiasa Y, Matsuda H, Tao M, Tsuzuki T, Hayashi I et al. (1995). Intratumor cellular heterogeneity and alterations in Ras oncogene and P53 tumor suppressor gene in human prostate carcinoma. Am J Pathol 147: 1112–1122.
Lapointe J, Li C, Higgins JP, van de RM, Bair E, Montgomery K et al. (2004). Gene expression profiling identifies clinically relevant subtypes of prostate cancer. Proc Natl Acad Sci USA 101: 811–816.
McNeal JE . (1988). Significance of duct-acinar dysplasia in prostatic carcinogenesis. Prostate 13: 91–102.
McNeal JE, Yemoto CE . (1996). Spread of adenocarcinoma within prostatic ducts and acini. Morphologic and clinical correlations. Am J Surg Pathol 20: 802–814.
Miller GJ, Cygan JM . (1994). Morphology of prostate cancer: the effects of multifocality on histological grade, tumor volume and capsule penetration. J Urol 152: 1709–1713.
Mirchandani D, Zheng J, Miller GJ, Ghosh AK, Shibata DK, Cote RJ et al. (1995). Heterogeneity in intratumor distribution of P53 mutations in human prostate cancer. Am J Pathol 147: 92–101.
Nam RK, Sugar L, Wang Z, Yang W, Kitching R, Klotz LH et al. (2007). Expression of TMPRSS2 ERG gene fusion in prostate cancer cells is an important prognostic factor for cancer progression. Cancer Biol Ther 6: 40–45.
Perner S, Demichelis F, Beroukhim R, Schmidt FH, Mosquera J-M, Setlur S et al. (2006). TMPRSS2:ERG fusion-associated deletions provide insight into the heterogeneity of prostate cancer. Cancer Res 66: 8337–8341.
Perner S, Mosquera JM, Demichelis F, Hofer MD, Paris PL, Simko J et al. (2007). TMPRSS2–ERG fusion prostate cancer: an early molecular event associated with invasion. Am J Surg Pathol. 31: 882–888.
Qian J, Bostwick DG, Takahashi S, Borell TJ, Herath JF, Lieber MM et al. (1995). Chromosomal anomalies in prostatic intraepithelial neoplasia and carcinoma detected by fluorescence in situ hybridization. Cancer Res 55: 5408–5414.
Radtke F, Clevers H . (2005). Self-renewal and cancer of the gut: two sides of a coin. Science 307: 1904–1909.
Sakai I, Harada K, Hara I, Eto H, Miyake H . (2005). A comparison of the biological features between prostate cancers arising in the transition and peripheral zones. BJU Int 96: 528–532.
Singh D, Febbo PG, Ross K, Jackson DG, Manola J, Ladd C et al. (2002). Gene expression correlates of clinical prostate cancer behavior. Cancer Cell 1: 203–209.
Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H et al. (2001). Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 98: 10869–10874.
Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A et al. (2003). Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA 100: 8418–8423.
Srigley JR, Amin MB, Bostwick DG, Grignon DJ, Hammond ME . (2000). Updated protocol for the examination of specimens from patients with carcinomas of the prostate gland: a basis for checklists. Cancer Committee. Arch Pathol Lab Med 124: 1034–1039.
Stephenson AJ, Smith A, Kattan MW, Satagopan J, Reuter VE, Scardino PT et al. (2005). Integration of gene expression profiling and clinical variables to predict prostate carcinoma recurrence after radical prostatectomy. Cancer 104: 290–298.
Tomlins SA, Mehra R, Rhodes DR, Smith LR, Roulston D, Helgeson BE et al. (2006). TMPRSS2: ETV4 gene fusions define a third molecular subtype of prostate cancer. Cancer Res 66: 3396–3400.
Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW et al. (2005). Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 310: 644–648.
Villers A, McNeal JE, Freiha FS, Stamey TA . (1992). Multiple cancers in the prostate. Morphologic features of clinically recognized versus incidental tumors. Cancer 70: 2313–2318.
Wang J, Cai Y, Ren C, Ittmann M . (2006). Expression of variant TMPRSS2/ERG fusion messenger RNAs is associated with aggressive prostate cancer. Cancer Res 66: 8347–8351.
Yoshimoto M, Joshua AM, Chilton-Macneill S, Bayani J, Selvarajah S, Evans AJ et al. (2006). Three-color FISH analysis of TMPRSS2/ERG fusions in prostate cancer indicates that genomic microdeletion of chromosome 21 is associated with rearrangement. Neoplasia 8: 465–469.
Yu YP, Landsittel D, Jing L, Nelson J, Ren B, Liu L et al. (2004). Gene expression alterations in prostate cancer predicting tumor aggression and preceding development of malignancy. J Clin Oncol 22: 2790–2799.
Acknowledgements
This work was funded by Cancer Research UK, the National Cancer Research Institute, the Grand Charity of Freemasons and the Rosetrees Trust. We thank Christine Bell for help with typing the manuscript. DMB is supported by The Orchid Appeal.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Supplementary information
Rights and permissions
About this article
Cite this article
Clark, J., Attard, G., Jhavar, S. et al. Complex patterns of ETS gene alteration arise during cancer development in the human prostate. Oncogene 27, 1993–2003 (2008). https://doi.org/10.1038/sj.onc.1210843
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210843
Keywords
This article is cited by
-
Experimental in vitro, ex vivo and in vivo models in prostate cancer research
Nature Reviews Urology (2023)
-
Identification and characterization of novel ETV4 splice variants in prostate cancer
Scientific Reports (2023)
-
C-MYC, HIF-1α, ERG, TKT, and GSTP1: an Axis in Prostate Cancer?
Pathology & Oncology Research (2019)
-
The prognostic utility of the transcription factor SRF in docetaxel-resistant prostate cancer: in-vitro discovery and in-vivo validation
BMC Cancer (2017)
-
The oncogene ERG: a key factor in prostate cancer
Oncogene (2016)