Abstract
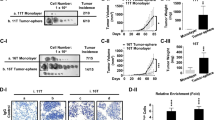
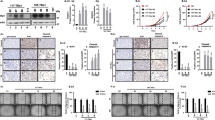
The molecular genetic events underlying thyroid carcinogenesis are not well understood. Mice harboring a dominant-negative mutant thyroid hormone receptor-β (TRβPV/PV mice) spontaneously develop follicular thyroid carcinoma similar to human cancer. The present study aimed to elucidate the role of the steroid receptor coactivator-3 (SRC-3) in thyroid carcinogenesis in vivo by using the offspring from the cross of TRβPV/PV and SRC-3−/− mice. TRβPV/PV mice deficient in SRC-3 (TRβPV/PVSRC-3−/− mice) had significantly increased survival, decreased thyroid tumor growth, delayed tumor progression and lower incidence of distant metastasis as compared with TRβPV/PV mice with SRC-3 (TRβPV/PVSRC-3+/+ mice). Further, in vivo and in vitro analyses of multiple signaling pathways indicated that SRC-3 deficiency could lead to (1) inhibition of cell cycle progression at the G1/S transition via controlling the expression of cell cycle regulators, such as E2F1; (2) induction of apoptosis by controlling the expression of the Bcl-2 and caspase-3 genes and (3) suppression of neovascularization and metastasis, at least in part, through modulating the vascular endothelial growth factor gene expression. Taken together, SRC-3 could play important roles through regulating multiple target genes and signaling pathways during thyroid carcinogenesis, understanding of which should direct future therapeutic options for thyroid cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY et al. (1997). AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science 277: 965–968.
Furumoto H, Ying H, Chandramouli GV, Zhao L, Walker RL, Meltzer PS et al. (2005). An unliganded thyroid hormone beta receptor activates the cyclin D1/cyclin-dependent kinase/retinoblastoma/E2F pathway and induces pituitary tumorigenesis. Mol Cell Biol 25: 124–135.
Gillenwater AM, Weber RS . (1997). Thyroid carcinoma. Cancer Treat Res 90: 149–169.
Kaneshige M, Kaneshige K, Zhu X, Dace A, Garrett L, Carter TA et al. (2000). Mice with a targeted mutation in the thyroid hormone beta receptor gene exhibit impaired growth and resistance to thyroid hormone. Proc Natl Acad Sci USA 97: 13209–13214.
Kato Y, Ying H, Willingham MC, Cheng SY . (2004). A tumor suppressor role for thyroid hormone beta receptor in a mouse model of thyroid carcinogenesis. Endocrinology 145: 4430–4438.
Kato Y, Ying H, Zhao L, Furuya F, Araki O, Willingham MC et al. (2006). PPARgamma insufficiency promotes follicular thyroid carcinogenesis via activation of the nuclear factor-kappaB signaling pathway. Oncogene 25: 2736–2747.
Kim CS, Ying H, Willingham MC, Cheng SY . (2007). The pituitary tumor transforming gene promotes angiogenesis in a mouse model of follicular thyroid cancer. Carcinogenesis 28: 932–939.
Liao L, Kuang SQ, Yuan Y, Gonzalez SM, O’Malley BW, Xu J . (2002). Molecular structure and biological function of the cancer-amplified nuclear receptor coactivator SRC-3/AIB1. J Steroid Biochem Mol Biol 83: 3–14.
Lonard DM, O’Malley BW . (2005). Expanding functional diversity of the coactivators. Trends Biochem Sci 30: 126–132.
Louie MC, Revenko AS, Zou JX, Yao J, Chen HW . (2006). Direct control of cell cycle gene expression by proto-oncogene product ACTR, and its autoregulation underlies its transforming activity. Mol Cell Biol 26: 3810–3823.
Louie MC, Zou JX, Rabinovich A, Chen HW . (2004). ACTR/AIB1 functions as an E2F1 coactivator to promote breast cancer cell proliferation and antiestrogen resistance. Mol Cell Biol 24: 5157–5171.
Meier CA, Dickstein BM, Ashizawa K, McClaskey JH, Muchmore P, Ransom SC et al. (1992). Variable transcriptional activity and ligand binding of mutant beta 1 3,5,3′-triiodothyronine receptors from four families with generalized resistance to thyroid hormone. Mol Endocrinol 6: 248–258.
Olateju TO, Vanderpump MP . (2006). Thyroid hormone resistance. Ann Clin Biochem 43: 431–440.
Parrilla R, Mixson AJ, McPherson JA, McClaskey JH, Weintraub BD . (1991). Characterization of seven novel mutations of the c-erbA beta gene in unrelated kindreds with generalized thyroid hormone resistance. Evidence for two ‘hot spot’ regions of the ligand binding domain. J Clin Invest 88: 2123–2130.
Suzuki H, Willingham MC, Cheng SY . (2002). Mice with a mutation in the thyroid hormone receptor beta gene spontaneously develop thyroid carcinoma: a mouse model of thyroid carcinogenesis. Thyroid 12: 963–969.
Torres-Arzayus MI, Font de Mora J, Yuan J, Vazquez F, Bronson R, Rue M et al. (2004). High tumor incidence and activation of the PI3K/AKT pathway in transgenic mice define AIB1 as an oncogene. Cancer Cell 6: 263–274.
Wu RC, Qin J, Hashimoto Y, Wong J, Xu J, Tsai SY et al. (2002). Regulation of SRC-3 (pCIP/ACTR/AIB-1/RAC-3/TRAM-1) Coactivator activity by I kappa B kinase. Mol Cell Biol 22: 3549–3561.
Xu J, Liao L, Ning G, Yoshida-Komiya H, Deng C, O’Malley BW . (2000). The steroid receptor coactivator SRC-3 (p/CIP/RAC3/AIB1/ACTR/TRAM-1) is required for normal growth, puberty, female reproductive function, and mammary gland development. Proc Natl Acad Sci USA 97: 6379–6384.
Yan J, Tsai SY, Tsai MJ . (2006a). SRC-3/AIB1: transcriptional coactivator in oncogenesis. Acta Pharmacol Sin 27: 387–394.
Yan J, Yu CT, Ozen M, Ittmann M, Tsai SY, Tsai MJ . (2006b). Steroid receptor coactivator-3 and activator protein-1 coordinately regulate the transcription of components of the insulin-like growth factor/AKT signaling pathway. Cancer Res 66: 11039–11046.
Ying H, Furuya F, Willingham MC, Xu J, O’Malley BW, Cheng SY . (2005). Dual functions of the steroid hormone receptor coactivator 3 in modulating resistance to thyroid hormone. Mol Cell Biol 25: 7687–7695.
Ying H, Suzuki H, Furumoto H, Walker R, Meltzer P, Willingham MC et al. (2003a). Alterations in genomic profiles during tumor progression in a mouse model of follicular thyroid carcinoma. Carcinogenesis 24: 1467–1479.
Ying H, Suzuki H, Zhao L, Willingham MC, Meltzer P, Cheng SY . (2003b). Mutant thyroid hormone receptor beta represses the expression and transcriptional activity of peroxisome proliferator-activated receptor gamma during thyroid carcinogenesis. Cancer Res 63: 5274–5280.
Zhou HJ, Yan J, Luo W, Ayala G, Lin SH, Erdem H et al. (2005). SRC-3 is required for prostate cancer cell proliferation and survival. Cancer Res 65: 7976–7983.
Acknowledgements
We thank B O’Malley for SRC-3 null mice and R Wu for the anti-SRC-3 antibodies. This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Supplementary information
Rights and permissions
About this article
Cite this article
Ying, H., Willingham, M. & Cheng, SY. The steroid receptor coactivator-3 is a tumor promoter in a mouse model of thyroid cancer. Oncogene 27, 823–830 (2008). https://doi.org/10.1038/sj.onc.1210680
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210680
Keywords
This article is cited by
-
Overexpression of steroid receptor coactivators alleviates hyperglycemia-induced endothelial cell injury in rats through activating the PI3K/Akt pathway
Acta Pharmacologica Sinica (2019)
-
Trichothecenes: immunomodulatory effects, mechanisms, and anti-cancer potential
Archives of Toxicology (2017)
-
Overexpression of AIB1 correlates inversely with E-cadherin expression in pancreatic adenocarcinoma and may promote lymph node metastasis
International Journal of Clinical Oncology (2014)
-
Up-regulation of nuclear receptor coactivator amplified in breast cancer-1 in papillary thyroid carcinoma correlates with lymph node metastasis
Clinical and Translational Oncology (2013)
-
Activation of tumor cell proliferation by thyroid hormone in a mouse model of follicular thyroid carcinoma
Oncogene (2012)