Abstract
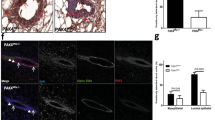
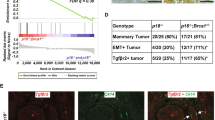
BRCA1 can regulate estrogen receptor-α (ERα) activity. This study tested the hypotheses that Brca1 loss in mammary epithelium alters the estrogenic growth response and that exposure to increased estrogen or ERα collaborates with Brca1 deficiency to accelerate preneoplasia and cancer development. Longer ductal extension was found in mammary glands of Brca1f/f;MMTV-Cre mice during puberty as compared to wild-type mice. Terminal end bud differentiation was impaired in Brca1 mutant mice with preservation of prolactin-induced alveolar differentiation. Exogenous estrogen stimulated an abnormal sustained increase in mammary epithelial cell proliferation and the appearance of ERα-negative preneoplasia in postpubertal Brca1 mutant mice. Carcinogenesis was investigated using Brca1f/f;MMTV-Cre mice hemizygous for p53. Exogenous estrogen increased the percentage of mice with multiple hyperplastic alveolar nodules. Targeted conditional ERα overexpression in mammary epithelial cells of mice that were Brca1 mutant and hemizygous for p53 increased the percentage of mice exhibiting multiple hyperplastic nodules, invasive mammary cancers and cancer multiplicity. Significantly more than half of the preneoplasia and cancers were ERα negative even as their initiation was promoted by ERα overexpression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Arbeit JM, Howley PM, Hanahan D . (1996). Chronic estrogen-induced cervical and vaginal squamous carcinogenesis in human papillomavirus type 16 transgenic mice. Proc Natl Acad Sci USA 93: 2930–2935.
Bachelier R, Xu X, Li C, Qiao W, Furth PA, Lubet RA et al. (2005). Effect of bilateral oophorectomy on mammary tumor formation in BRCA1 mutant mice. Oncol Rep 14: 1117–1120.
Bocchinfuso WP, Korach KS . (1997). Mammary gland development and tumorigenesis in estrogen receptor knockout mice. J Mammary Gland Biol Neoplasia 2: 323–334.
Brodie SG, Xu X, Qiao W, Li WM, Cao L, Deng CX . (2001). Multiple genetic changes are associated with mammary tumorigenesis in Brca1 conditional knockout mice. Oncogene 20: 7514–7523.
Cui X, Lazard Z, Zhang P, Hopp TA, Lee AV . (2003). Progesterone crosstalks with insulin-like growth factor signaling in breast cancer cells via induction of insulin receptor substrate-2. Oncogene 22: 6937–6941.
Cullinane CA, Lubinski J, Neuhausen SL, Ghadirian P, Lynch HT, Isaacs C et al. (2005). Effect of pregnancy as a risk factor for breast cancer in BRCA1/BRCA2 mutation carriers. Int J Cancer 117: 988–991.
Deans AJ, Simpson KJ, Trivett MK, Brown MA, McArthur GA . (2004). Brca1 inactivation induces p27(Kip1)-dependent cell cycle arrest and delayed development in the mouse mammary gland. Oncogene 23: 6136–6145.
Decensi A, Serrano D, Bonanni B, Cazzaniga M, Guerrieri-Gonzaga A . (2003). Breast cancer prevention trials using retinoids. J Mammary Gland Biol Neoplasia 8: 19–30.
Dembinski T, Shiu R . (1987). Growth factors in mammary gland development and function. In: Neville, MC and Daniel CW (eds.) The Mammary Gland: Development, Regulation, and Function. Plenum Pub Corp: USA, pp 355–381.
El Etreby MF, Liang Y, Wrenn RW, Schoenlein PV . (1998). Additive effect of mifepristone and tamoxifen on apoptotic pathways in MCF-7 human breast cancer cells. Breast Cancer Res Treat 51: 149–168.
Fan S, Ma YX, Wang C, Yuan RQ, Meng Q, Wang JA et al. (2002). p300 modulates the BRCA1 inhibition of estrogen receptor activity. Cancer Res 62: 141–151.
Fendrick JL, Raafat AM, Haslam SZ . (1998). Mammary gland growth and development from the postnatal period to postmenopause: ovarian steroid receptor ontogeny and regulation in the mouse. J Mammary Gland Biol Neoplasia 3: 7–22.
Foulkes WD, Metcalfe K, Sun P, Hanna WM, Lynch HT, Ghadirian P et al. (2004). Estrogen receptor status in BRCA1- and BRCA2-related breast cancer: the influence of age, grade, and histological type. Clin Cancer Res 10: 2029–2034.
Frech MS, Halama ED, Tilli MT, Singh B, Gunther EJ, Chodosh LA et al. (2005a). Deregulated estrogen receptor alpha expression in mammary epithelial cells of transgenic mice results in the development of ductal carcinoma in situ. Cancer Res 65: 681–685.
Frech MS, Jones LP, Furth PA . (2005b). Validation of transgenic models of breast cancer: ductal carcinoma in situ (DCIS) and Brca1 mutation-related breast cancer. Breast Cancer Online 8. http://www.bco.org/article.asp?article=273&issue=78.
Furuta S, Jiang X, Gu B, Cheng E, Chen PL, Lee WH . (2005). Depletion of BRCA1 impairs differentiation but enhances proliferation of mammary epithelial cells. Proc Natl Acad Sci USA 102: 9176–9181.
Furuta S, Wang JM, Wei S, Jeng YM, Jiang X, Gu B et al. (2006). Removal of BRCA1/CtIP/ZBRK1 repressor complex on ANG1 promoter leads to accelerated mammary tumor growth contributed by prominent vasculature. Cancer Cell 10: 13–24.
Ginsburg E, Vonderhaar B . (2000) In: Ip M and Asch B (eds.) Methods in Mammary Gland Biology and Breast Cancer Research. Kluwer Academic/Plenum Publishers: NYC, pp 147–154.
Grimm SL, Rosen JM . (2006). Stop! In the name of transforming growth factor-beta: keeping estrogen receptor-alpha-positive mammary epithelial cells from proliferating. Breast Cancer Res 8: 106.
Gudmundsdottir K, Ashworth A . (2006). The roles of BRCA1 and BRCA2 and associated proteins in the maintenance of genomic stability. Oncogene 25: 5864–5874.
Heppner GH, Miller FR, Shekhar PM . (2000). Nontransgenic models of breast cancer. Breast Cancer Res 2: 331–334.
Herschkowitz JI, Simin K, Weigman VJ, Mikaelian I, Usary J, Hu Z et al. (2007). Identification of conserved gene expression features of murine breast carcinoma models relative to each other and to human cancers. Genome Biol 8: R76 (Epub ahead of print).
Jones LP, Li M, Halama ED, Ma Y, Lubet R, Grubbs CJ et al. (2005). Promotion of mammary cancer development by temoxifen in a mouse model of Brca1-mutation-related breast cancer. Oncogene 24: 3554–3562.
Krzysiek J, Milewicz T, Augustowska K, Sztefko K, Rys J, Zubel A et al. (2003). The impact of progesterone on simultaneous, local secretion of IGFBP-3 and IGF-I [IGFBP-3/IGF-I index] by human malignant and non-malignant breast explants depends on tissue steroid receptor phenotype. Ginekol Pol 74: 767–774.
Liang Y, Hou M, Kallab AM, Barrett JT, El Etreby F, Schoenlein PV . (2003). Induction of antiproliferation and apoptosis in estrogen receptor negative MDA-231 human breast cancer cells by mifepristone and 4-hydroxytamoxifen combination therapy: a role for TGFbeta. Int J Oncol 23: 369–380.
Ma Y, Katiyar P, Jones LP, Fan S, Zhang Y, Furth PA et al. (2006). The breast cancer susceptibility gene BRCA1 regulates progesterone receptor signaling in mammary epithelial cells. Mol Endocrinol 20: 14–34.
Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S et al. (1994). A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 266: 66–71.
Milewicz T, Gregoraszczuk EL, Sztefko K, Augustowska K, Krzysiek J, Rys J . (2005). Lack of synergy between estrogen and progesterone on local IGF-I, IGFBP-3 and IGFBP-2 secretion by both hormone-dependent and hormone-independent breast cancer explants in vitro. Effect of tamoxifen and mifepristone (RU 486). Growth Horm IGF Res 15: 140–147.
Poole AJ, Li Y, Kim Y, Lin S-CJ, Lee W-H, Lee EYHP . (2006). Prevention of Brca1-mediated mammary tumorigenesis in mice by a progesterone antagonist. Science 314: 1467–1470.
Raafat AM, Hofseth LJ, Li S, Bennett JM, Haslam SZ . (1999). A mouse model to study the effects of hormone replacement therapy on normal mammary gland during menopause: enhanced proliferative response to estrogen in late postmenopausal mice. Endocrinology 140: 2570–2580.
Razandi M, Pedram A, Rosen EM, Levin ER . (2004). BRCA1 inhibits membrane estrogen and growth factor receptor signaling to cell proliferation in breast cancer. Mol Cell Biol 24: 5900–5913.
Rebbeck TR, Lynch HT, Neuhausen SL, Narod SA, Van't Veer L, Garber JE et al. (2002). Prophylactic oophorectomy in BRCA1 and BRCA2 mutation carriers. N Engl J Med 346: 1616–1622.
Ruan W, Monaco ME, Kleinberg DL . (2005). Progesterone stimulates mammary gland ductal morphogenesis by synergizing with and enhancing insulin-like growth factor-I action. Endocrinology 146: 1170–1178.
Shukla V, Coumoul X, Cao L, Wang RH, Xiao C, Xu X et al. (2006). Absence of the full-length breast cancer-associated gene-1 leads to increased expression of insulin-like growth factor signaling axis members. Cancer Res 66: 7151–7157.
Silberstein GB . (2001). Postnatal mammary gland morphogenesis. Microsc Res Tech 52: 155–162.
Tilli MT, Frech MS, Steed ME, Hruska KS, Johnson MD, Flaws JA et al. (2003). Introduction of estrogen receptor-alpha into the tTA/TAg conditional mouse model precipitates the development of estrogen-responsive mammary adenocarcinoma. Am J Pathol 163: 1713–1719.
Vassen L, Wegrzyn W, Klein-Hitpass L . (1999). Human insulin receptor substrate-2 (IRS-2) is a primary progesterone response gene. Mol Endocrinol 13: 485–494.
Wagner KU, McAllister K, Ward T, Davis B, Wiseman R, Hennighausen L . (2001). Cre-mediated gene deletion in the mammary gland. Transgenic Res 10: 545–553.
Xu X, Wagner KU, Larson D, Weaver Z, Li C, Ried T et al. (1999). Conditional mutation of Brca1 in mammary epithelial cells results in blunted ductal morphogenesis and tumour formation. Nat Genet 22: 37–43.
Acknowledgements
This study was supported by NIH NCI MAO/RFP NO1-CN-05024 (PAF and LPJ), RO1CA08000 (EMR, PAF and KT), 2T32CA09686-08 (LPJ) and the Susan G Komen Breast Cancer Foundation PDF040244 (LPJ and SA) and PDF0503642 (MTT).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Supplementary information
Rights and permissions
About this article
Cite this article
Jones, L., Tilli, M., Assefnia, S. et al. Activation of estrogen signaling pathways collaborates with loss of Brca1 to promote development of ERα-negative and ERα-positive mammary preneoplasia and cancer. Oncogene 27, 794–802 (2008). https://doi.org/10.1038/sj.onc.1210674
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210674
Keywords
This article is cited by
-
Expression and activation of nuclear hormone receptors result in neuronal differentiation and favorable prognosis in neuroblastoma
Journal of Experimental & Clinical Cancer Research (2022)
-
Disruption of STAT5A and NMI signaling axis leads to ISG20-driven metastatic mammary tumors
Oncogenesis (2021)
-
Environmental exposures during windows of susceptibility for breast cancer: a framework for prevention research
Breast Cancer Research (2019)
-
BRCA1-mimetic compound NSC35446.HCl inhibits IKKB expression by reducing estrogen receptor-α occupancy in the IKKB promoter and inhibits NF-κB activity in antiestrogen-resistant human breast cancer cells
Breast Cancer Research and Treatment (2017)
-
MicroRNA-206 is differentially expressed in Brca1-deficient mice and regulates epithelial and stromal cell compartments of the mouse mammary gland
Oncogenesis (2016)