Abstract
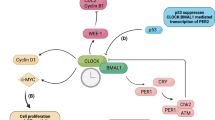
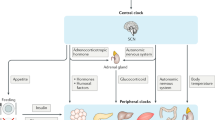
Circadian rhythms regulate diverse physiological processes including homeostatic functions of steroid hormones and their receptors. Estrogen receptor-α (ERα) is essential for normal mammary gland physiology and is a prognostic marker for the treatment of breast cancer. We report that Per2, a core clock gene, links the circadian cycle to the ERα signaling network. Binding of enhances ERα degradation, while suppression of Per2 levels leads to ERα stabilization. In turn, Per2 itself is estrogen inducible in these cells, suggesting a feedback mechanism to attenuate stimulation by estrogen. In addition, overexpression of Per2 in breast cancer cells leads to significant growth inhibition, loss of clonogenic ability and apoptosis. Taken together, these results further support a critical role for peripheral circadian regulation in tissue homeostasis and suggest a novel role for clock genes in estrogen receptor-positive breast cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Chen ST, Choo KB, Hou MF, Yeh KT, Kuo SJ, Chang JG . (2005). Deregulated expression of the PER1, PER2 and PER3 genes in breast cancers. Carcinogenesis 26: 1241–1246.
Fu L, Pelicano H, Liu J, Huang P, Lee C . (2002). The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell 111: 41–50.
Gallego M, Virshup DM . (2007). Post-translational modifications regulate the ticking of the circadian clock. Nat Rev Mol Cell Biol 8: 139–148.
Hansen J . (2001). Light at night, shiftwork, and breast cancer risk. J Natl Cancer Inst 93: 1513–1515.
Hua H, Wang Y, Wan C, Liu Y, Zhu B, Yang C et al. (2006). Circadian gene mPer2 overexpression induces cancer cell apoptosis. Cancer Sci 97: 589–596.
Ishida N . (2007). Circadian clock, cancer and lipid metabolism. Neurosci Res 57: 483–490.
Ko CH, Takahashi JS . (2006). Molecular components of the mammalian circadian clock. Hum Mol Genet 20062: R271–R277.
Lowrey PL, Takahashi JS . (2004). Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Annu Rev Genomics Hum Genet 5: 407–441.
Matsuo T, Yamaguchi S, Mitsui S, Emi A, Shimoda F, Okamura H . (2003). Control mechanism of the circadian clock for timing of cell division in vivo. Science 302: 255–259.
McDonnell DP, Norris JD . (2002). Connections and regulation of the human estrogen receptor. Science 296: 1642–1644.
Metz RP, Qu X, Laffin B, Earnest D, Porter WW . (2006). Circadian clock and cell cycle gene expression in mouse mammary epithelial cells and in the developing mouse mammary gland. Dev Dyn 235: 263–271.
Miller BH, McDearmon EL, Panda S, Hayes KR, Zhang J, Andrews JL et al. (2007). Circadian and CLOCK-controlled regulation of the mouse transcriptome and cell proliferation. Proc Natl Acad Sci USA 104: 3342–3347.
Nakamura TJ, Moriya T, Inoue S, Shimazoe T, Watanabe S, Ebihara S et al. (2005). Estrogen differentially regulates expression of Per1 and Per2 genes between central and peripheral clocks and between reproductive and nonreproductive tissues in female rats. J Neurosci Res 82: 622–630.
Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M et al. (2002). Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 109: 307–320.
Perrin JS, Segall LA, Harbour VL, Woodside B, Amir S . (2006). The expression of the clock protein PER2 in the limbic forebrain is modulated by the estrous cycle. Proc Natl Acad Sci USA 103: 5591–5596.
Reid G, Denger S, Kos M, Gannon F . (2002). Human estrogen receptor-alpha: regulation by synthesis, modification and degradation. Cell Mol Life Sci 59: 821–831.
Reppert SM, Weaver DR . (2002). Coordination of circadian timing in mammals. Nature 418: 935–941.
Romagnolo D, Annab LA, Thompson TE, Risinger JI, Terry LA, Barrett JC et al. (1998). Estrogen upregulation of BRCA1 expression with no effect on localization. Mol Carcinog 22: 102–109.
Schibler U, Sassone-Corsi P . (2002). A web of circadian pacemakers. Cell 111: 919–922.
Shearman LP, Sriram S, Weaver DR, Maywood ES, Chaves I, Zheng B et al. (2000). Interacting molecular loops in the mammalian circadian clock. Science 288: 1013–1019.
Shi Y, Downes M, Xie W, Kao HY, Ordentlich P, Tsai CC et al. (2001). Sharp, an inducible cofactor that integrates nuclear receptor repression and activation. Genes Dev 15: 1140–1151.
Sternlicht MD . (2006). Key stages in mammary gland development: the cues that regulate ductal branching morphogenesis. Breast Cancer Res 8: 201.
Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH . (2002). Extensive and divergent circadian gene expression in liver and heart. Nature 417: 78–83.
Yager JD, Davidson NE . (2006). Estrogen carcinogenesis in breast cancer. N Engl J Med 354: 270–282.
Yagita K, Tamanini F, Yasuda M, Hoeijmakers JH, van der Horst GT, Okamura H . (2002). Nucleocytoplasmic shuttling and mCRY-dependent inhibition of ubiquitylation of the mPER2 clock protein. EMBO J 21: 1301–1314.
Yang X, Downes M, Yu RT, Bookout AL, He W, Straume M et al. (2006). Nuclear receptor expression links the circadian clock to metabolism. Cell 126: 801–810.
Acknowledgements
This work was supported by NIH grants, UCLA Cancer Gene Medicine Training grant and also in part by the Parker Hughes Trust, the Inger Foundation and the Mary Barry Foundation. H Phillip Koeffler is a member of the UCLA Jonsson Comprehensive Cancer Center and holds the endowed Mark Goodson Chair of Oncology Research at Cedars-Sinai Medical Center/UCLA School of Medicine.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Supplementary information
Rights and permissions
About this article
Cite this article
Gery, S., Virk, R., Chumakov, K. et al. The clock gene Per2 links the circadian system to the estrogen receptor. Oncogene 26, 7916–7920 (2007). https://doi.org/10.1038/sj.onc.1210585
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210585
Keywords
This article is cited by
-
Obesity, cancer risk, and time-restricted eating
Cancer and Metastasis Reviews (2022)
-
Clocking cancer: the circadian clock as a target in cancer therapy
Oncogene (2021)
-
Circadian rhythms: influence on physiology, pharmacology, and therapeutic interventions
Journal of Pharmacokinetics and Pharmacodynamics (2021)
-
Metabolic rivalry: circadian homeostasis and tumorigenesis
Nature Reviews Cancer (2020)
-
Timing gone awry: distinct tumour suppressive and oncogenic roles of the circadian clock and crosstalk with hypoxia signalling in diverse malignancies
Journal of Translational Medicine (2019)