Abstract
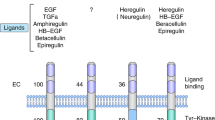
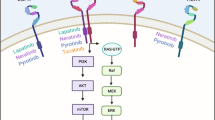
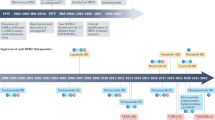
The human epidermal growth factor receptor 2 (HER2) tyrosine kinase receptor is overexpressed in approximately 20–30% of human breast cancers, and is associated with reduced survival. Hence, numerous therapeutic strategies have been tested for their ability to target the HER2 protein. The humanized monoclonal antibody trastuzumab (Herceptin) was the first HER2-targeted agent approved for clinical use in breast cancer patients. Response rates to single-agent trastuzumab range from 12 to 34% for metastatic breast cancer (MBC), and significant improvements in survival rates are achieved in patients with early-stage HER2-overexpressing breast cancer in the adjuvant setting. Despite its initial efficacy, acquired resistance to trastuzumab develops in a majority of patients with MBC, and a large subset never responds, demonstrating primary resistance. Molecular mechanisms of trastuzumab antineoplastic activity and potential mechanisms contributing to its resistance will be discussed in this review. Novel agents that may enhance trastuzumab efficacy will also be discussed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Adams CW, Allison DE, Flagella K, Presta L, Clarke J, Dybdal N et al. (2006). Humanization of a recombinant monoclonal antibody to produce a therapeutic HER dimerization inhibitor, pertuzumab. Cancer Immunol Immunother 55: 717–727.
Agus DB, Akita RW, Fox WD, Lewis GD, Higgins B, Pisacane PI et al. (2002). Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell 2: 127–137.
Ali SM, Esteva FJ, Fornier M, Gligorov J, Harris L, Kostler WJ et al. (2006). Serum HER-2/neu change predicts clinical outcome to trastuzumab-based therapy. J Clin Oncol 24 (June 20 Supplement): 500.
Anido J, Scaltriti M, Bech Serra JJ, Santiago Josefat B, Todo FR, Baselga J et al. (2006). Biosynthesis of tumorigenic HER2 C-terminal fragments by alternative initiation of translation. EMBO 25: 3234–3244.
Arnould L, Gelly M, Penault-Llorca F, Benoit L, Bonnetain F, Migeon C et al. (2006). Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism? Br J Cancer 94: 259–267.
Baselga J, Tripathy D, Mendelsohn J, Baughman S, Benz CC, Dantis L et al. (1996). Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J Clin Oncol 14: 737–744.
Baselga J, Albanell J, Molina MA, Arribas J . (2001). Mechanism of action of trastuzumab and scientific update. Semin Oncol 28 (5 Suppl 16): 4–11.
Burstein HJ, Lieberman G, Slamon DJ, Winer EP, Klein P . (2005). Isolated central nervous system metastases in patients with HER2-overexpressing advanced breast cancer treated with first-line trastuzumab-based therapy. Ann Oncol 16: 1772–1777.
Buzdar AU, Ibrahim NK, Francis D, Booser DJ, Thomas ES, Theriault RL et al. (2005). Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol 23: 3676–3685.
Camirand A, Lu Y, Pollak M . (2002). Co-targeting HER2/ErbB2 and insulin-like growth factor-1 receptors causes synergistic inhibition of growth in HER2-overexpressing breast cancer cells. Med Sci Monit 8: BR521–BR526.
Carter P, Presta L, Gorman CM, Ridgway JB, Henner D, Wong WL et al. (1992). Humanization of an anti-p185her2 antibody for human cancer therapy. Proc Natl Acad Sci USA 89: 4285–4289.
Christianson TA, Doherty JK, Lin YJ, Ramsey EE, Holmes R, Keenan EJ et al. (1998). NH2-terminally truncated HER-2/neu protein: relationship with shedding of the extracellular domain and with prognostic factors in breast cancer. Cancer Res 58: 5123–5129.
Clynes RA, Towers TL, Presta LG, Ravetch JV . (2000). Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med 6: 443–446.
Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L et al. (1999). Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER-2 overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 17: 2639–2648.
Colomer R, Montero S, Lluch A, Ojeda B, Barnadas A, Casado A et al. (2000). Circulating HER2 extracellular domain and resistance to chemotherapy in advanced breast cancer. Clin Cancer Res 6: 2356–2362.
Cooley S, Burns LJ, Repka T, Miller JS . (1999). Natural killer cell cytotoxicity of breast cancer targets is enhanced by two distinct mechanisms of antibody-dependent cellular cytotoxicity against LFA-3 and HER2/neu. Exp Hematol 27: 1533–1541.
DiGiovanna MP, Chakraborty A . (2006). Combinations of HER2, estrogen receptor (ER) and IGF-I receptor (IGF1R) inhibitors induce apoptosis in breast cancer cells: Dramatic effects of HER2 inhibitors on non-overexpressing cells. Proc Am Assoc Cancer Res 47: 1226.
Esteva FJ, Cheli CD, Fritsche H, Fornier M, Slamon D, Thiel RP et al. (2005). Clinical utility of serum HER2/neu in monitoring and prediction of progression-free survival in metastatic breast cancer patients treated with trastuzumab-based therapies. Breast Cancer Res 7: R436–R443.
Esteva FJ, Valero V, Booser D, Guerra LT, Murray JL, Pusztai L et al. (2002). Phase II study of weekly docetaxel and trastuzumab for patients with HER-2-overexpressing metastatic breast cancer. J Clin Oncol 20: 1800–1808.
Franklin MC, Carey KD, Vajdos FF, Leahy DJ, de Vos AM, Sliwkowski MX . (2004). Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell 5: 317–328.
Gennari R, Menard S, Fagnoni F, Ponchio L, Scelsi M, Tagliabue E et al. (2004). Pilot study of the mechanism of action of preoperative trastuzumab in patients with primary operable breast tumors overexpressing HER2. Clin Cancer Res 10: 5650–5655.
Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T et al. (2006). Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 355: 2733–2743.
Graus-Porta D, Beerli RR, Daly JM, Hynes NE . (1997). ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO 16: 1647–1655.
Hayes DF, Yamauchi H, Broadwater G, Cirrincione CT, Rodrigue SP, Berry DA et al. (2001). Circulating HER-2/erbB-2/c-neu (HER-2) extracellular domain as a prognostic factor in patients with metastatic breast cancer: Cancer and Leukemia Group B Study 8662. Clin Cancer Res 7: 2703–2711.
Izumi Y, Xu L, di Tomaso E, Fukumura D, Jain RK . (2002). Tumour biology: Herceptin acts as an anti-angiogenic cocktail. Nature 416: 279–280.
Jerome L, Alami N, Belanger S, Page V, Yu Q, Paterson J et al. (2006). Recombinant human insulin-like growth factor binding protein 3 inhibits growth of human epidermal growth factor receptor-2-overexpressing breast tumors and potentiates herceptin activity in vivo. Cancer Res 66: 7245–7252.
Klos KS, Zhou X, Lee S, Zhang L, Yang W, Nagata Y et al. (2003). Combined trastuzumab and paclitaxel treatment better inhibits ErbB-2-mediated angiogenesis in breast carcinoma through a more effective inhibition of Akt than either treatment alone. Cancer 98: 1377–1385.
Konecny GE, Pegram MD, Venkatesan N, Finn R, Yang G, Rahmeh M et al. (2006). Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res 66: 1630–1639.
Kostler WJ, Schwab B, Singer CF, Neumann R, Rucklinger E, Brodowicz T . (2004). Monitoring of serum Her-2/neu predicts response and progression-free survival to trastuzumab-based treatment in patients with metastatic breast cancer. Clin Cancer Res 10: 1618–1624.
Lane HA, Beuvink I, Motoyama AB, Daly JM, Neve RM, Hynes NE . (2000). ErbB2 potentiates breast tumor proliferation through modulation of p27(Kip1)-Cdk2 complex formation: receptor overexpression does not determine growth dependency. Mol Cell Biol 20: 3210–3223.
Le XF, Claret FX, Lammayot A, Tian L, Deshpande D, LaPushin R et al. (2003). The role of cyclin-dependent kinase inhibitor p27Kip1 in anti-HER2 antibody-induced G1 cell cycle arrest and tumor growth inhibition. J Biol Chem 278: 23441–23450.
Leitzel K, Teramoto Y, Konrad K, Chinchilli VM, Volas G, Grossberg H et al. (1995). Elevated serum c-erbB-2 antigen levels and decreased response to hormone therapy of breast cancer. J Clin Oncol 13: 1129–1135.
Lin YZ, Clinton GM . (1991). A soluble protein related to the HER-2 proto-oncogene product is released from human breast carcinoma cells. Oncogene 6: 639–643.
Lu Y, Zi X, Pollak M . (2004). Molecular mechanisms underlying IGF-I-induced attenuation of the growth-inhibitory activity of trastuzumab (Herceptin) on SKBR3 breast cancer cells. Int J Cancer 108: 334–341.
Lu Y, Zi X, Zhao Y, Mascarenhas D, Pollak M . (2001). Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin). J Natl Cancer Inst 93: 1852–1857.
Marty M, Cognetti F, Maraninchi D, Snyder R, Mauriac L, Tubiana-Hulin M et al. (2005). Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol 23: 4265–4274.
Molina MA, Codony-Servat J, Albanell J, Rojo F, Arribas J, Baselga J . (2001). Trastuzumab (Herceptin), a humanized anti-HER2 receptor monoclonal antibody, inhibits basal and activated HER2 ectodomain cleavage in breast cancer cells. Cancer Res 61: 4744–4749.
Molina MA, Saez R, Ramsey EE, Garcia-Barchino MJ, Rojo F, Evans AJ et al. (2002). NH2-terminal truncated HER-2 protein but not full-length receptor is associated with nodal metastasis in human breast cancer. Clin Cancer Res 8: 347–353.
Nahta R, Esteva FJ . (2006). Herceptin: mechanisms of action and resistance. Cancer Lett 232: 123–138.
Nahta R, Hung MC, Esteva FJ . (2004a). The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res 64: 2343–2346.
Nahta R, Takahashi T, Ueno NT, Hung MC, Esteva FJ . (2004b). P27 (kip1) down-regulation is associated with trastuzumab resistance in breast cancer cells. Cancer Res 64: 3981–3986.
Nahta R, Yu D, Hung MC, Hortobagyi GN, Esteva FJ . (2006). Mechanisms of Disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol 3: 269–280.
Nahta R, Yuan LX, Zhang B, Kobayashi R, Esteva FJ . (2005). Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res 65: 11118–11128.
Neve RM, Sutterluty H, Pullen N, Lane HA, Daly JM, Krek W et al. (2000). Effects of oncogenic ErbB2 on G1 cell cycle regulators in breast tumour cells. Oncogene 19: 1647–1656.
Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I et al. (2005). Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353: 1659–1672.
Press MF, Bernstein L, Thomas PA, Meisner LF, Zhou JY, Ma Y et al. (1997). HER-2/neu gene amplification characterized by fluorescence in situ hybridization: poor prognosis in node-negative breast carcinomas. J Clin Oncol 15: 2894–2904.
Pupa SM, Menard S, Morelli D, Pozzi B, De Palo G, Colnaghi MI . (1993). The extracellular domain of the c-erbB-2 oncoprotein is released from tumor cells by proteolytic cleavage. Oncogene 8: 2917–2923.
Romond EH, Perez EA, Bryant J, Suman VJ, Geyer Jr CE, Davidson NE et al. (2005). Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353: 1673–1684.
Seidman AD, Fornier MN, Esteva FJ, Tan L, Kaptain S, Bach A et al. (2001). Weekly trastuzumab and paclitaxel therapy for metastatic breast cancer with analysis of efficacy by HER2 immunophenotype and gene amplification. J Clin Oncol 19: 2587–2595.
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL . (1987). Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235: 177–182.
Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE et al. (1989). Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244: 707–712.
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A et al. (2001). Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344: 783–792.
Sliwkowski MX, Lofgren JA, Lewis GD, Hotaling TE, Fendly BM, Fox JA . (1999). Nonclinical studies addressing the mechanism of action of trastuzumab (Herceptin). Semin Oncol 26 (4 Suppl 12): 60–70.
Tanner M, Kapanen AI, Junttila T, Raheem O, Grenman S, Elo J et al. (2004). Characterization of a novel cell line established from a patient with Herceptin-resistant breast cancer. Mol Cancer Ther 3: 1585–1592.
Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L et al. (2002). Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol 20: 719–726.
Wen XF, Yang G, Mao W, Thornton A, Liu J, Bast RC et al. (2006). HER2 signaling modulates the equilibrium between pro- and antiangiogenic factors via distinct pathways: implications for HER2-targeted antibody therapy. Oncogene 25: 6986–6996.
Xia W, Mullin RJ, Keith BR, Liu LH, Ma H, Rusnak DW et al. (2002). Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene 21: 6255–6263.
Xia W, Gerard CM, Liu L, Baudson NM, Ory TL, Spector NL . (2005). Combining lapatinib (GW572016), a small molecule inhibitor of ErbB1 and ErbB2 tyrosine kinases, with therapeutic anti-ErbB2 antibodies enhances apoptosis of ErbB2-overexpressing breast cancer cells. Oncogene 24: 6213–6221.
Yakes FM, Chinratanalab W, Ritter CA, King W, Seelig S, Arteaga CL . (2002). Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res 62: 4132–4141.
Yamauchi H, O'Neill A, Gelman R, Carney W, Tenney DY, Hosch S et al. (1997). Prediction of response to antiestrogen therapy in advanced breast cancer patients by pretreatment circulating levels of extracellular domain of the HER-2/c-neu protein. J Clin Oncol 15: 2518–2525.
Zabrecky JR, Lam T, McKenzie SJ, Carney W . (1991). The extracellular domain of p185/neu is released from the surface of human breast carcinoma cells, sk-br-3. J Biol Chem 266: 1716–1720.
Acknowledgements
We thank funding from the National Cancer Institute (K01CA118174, R Nahta), the Breast Cancer Research Foundation (FJ Esteva), the University Cancer Foundation at the University of Texas MD Anderson Cancer Center (FJ Esteva, R Nahta), the Nellie B Connally Breast Cancer Research Fund, and NIH Cancer Center Support Grant CA-16672 (Media Preparation Facility and Flow Cytometry facilities).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Nahta, R., Esteva, F. Trastuzumab: triumphs and tribulations. Oncogene 26, 3637–3643 (2007). https://doi.org/10.1038/sj.onc.1210379
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210379
Keywords
This article is cited by
-
Anti-tumor antibody isotype response can be modified with locally administered immunoadjuvants
Drug Delivery and Translational Research (2023)
-
Safety and efficacy profile of Trastuzumab deruxtecan in solid cancer: pooled reanalysis based on clinical trials
BMC Cancer (2022)
-
Hyperpolarized [1-13C]Pyruvate Magnetic Resonance Spectroscopic Imaging for Evaluation of Early Response to Tyrosine Kinase Inhibition Therapy in Gastric Cancer
Molecular Imaging and Biology (2022)
-
When the MET receptor kicks in to resist targeted therapies
Oncogene (2021)
-
Multiplexed live-cell profiling with Raman probes
Nature Communications (2021)