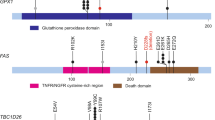
Abstract
Homozygous deletions (HD) provide an important resource for identifying the location of candidate tumor suppressor genes. To identify the tumor suppressor gene in oral cancer, we employed high-resolution comparative genomic hybridization (CGH)-array analysis. We identified a homozygous loss of FAT (4q35), a new member of the human cadherin superfamily, from genome-wide screening of copy number alterations in one primary oral cancer. This result was evaluated by genomic polymerase chain reaction in 13 oral cancer cell lines and 20 primary oral cancers and Southern blot in the cell lines. We found frequent exonic HD of FAT in the cell lines (3/13, 23%) and in primary oral cancers (16/20, 80%). FAT expression was absent in these cell lines. Homozygous deletion hot spots were observed in exon 1 (9/20, 45%) and exon 4 (7/20, 35%). Moreover, loss of gene expression was identified in other types of squamous cell carcinoma. The methylation status of the FAT CpG island in squamous cell carcinomas correlated negatively with its expression. Our results identify mutations in FAT as an important factor in the development of oral cancer and indicate the importance of FATs function in some squamous cell carcinomas.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Abbreviations
- OSCC:
-
oral squamous cell carcinoma
- HD:
-
homozygous deletion
- LOH:
-
loss of heterozygosity
- FISH:
-
fluorescence in situ hybridization
- CGH:
-
comparative genomic hybridization
- BAC:
-
bacterial artificial chromosome
- LCM:
-
laser-capture microdissection
- RT-PCR:
-
reverse transcriptase-polymerase chain reaction
- MSP:
-
methylation-specific PCR
- DMSO:
-
dimethyl sulfoxide
References
Beder LB, Gunduz M, Ouchida M, Fukushima K, Gunduz E, Ito S et al. (2003). Genome-wide analyses on loss of heterozygosity in head and neck squamous cell carcinomas. Lab Invest 83: 99–105.
Bryant PJ, Huettner B, Held Jr LI, Ryerse J, Szidonya J . (1988). Mutations at the fat locus interfere with cell proliferation control and epithelial morphogenesis in Drosophila. Dev Biol 129: 541–554.
Dryja TP, Cavenee W, White R, Rapaport JM, Peterson R, Albert DM et al. (1984). Homozygosity of chromosome 13 in retinoblastoma. N Engl J Med 310: 550–553.
Frijters ACJ, Zhang Z, van Damme M, Wang GL, Ronald PC, Michelmore RW . (1997). Construction of a bacterial artificial chromosome library containing large EcoRI and HindIII genomic fragments of lettuce. Theor Appl Genet 94: 390–399.
Garoia F, Guerra D, Pezzoli MC, Lopez-Varea A, Cavicchi S, Garcia-Bellido A . (2000). Cell behaviour of Drosophila fat cadherin mutations in wing development. Mech Dev 94: 95–109.
Haughey BH, Von Hoff DD, Windle BE, Wahl GM, Mock PM . (1992). c-myc Oncogene copy number in squamous carcinoma of the head and neck. Am J Otolaryngol 13: 168–171.
Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB . (1996). Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA 93: 9821–9826.
Hodgson G, Hager JH, Volik S, Hariono S, Wernick M, Moore D et al. (2001). Genome scanning with array CGH delineates regional alterations in mouse islet carcinomas. Nat Genet 29: 459–464.
IARC. GLOBOCAN, 2002 (2004). web-page address at http://www-dep.iarc.fr.
Ishwad CS, Shuster M, Bockmuhl U, Thakker N, Shah P, Toomes C et al. (1999). Frequent allelic loss and homozygous deletion in chromosome band 8p23 in oral cancer. Int J Cancer 80: 25–31.
Keller G, Vogelsang H, Becker I, Hutter J, Ott K, Candidus S et al. (1999). Diffuse type gastric and lobular breast carcinoma in a familial gastric cancer patient with an E-cadherin germline mutation. Am J Pathol 155: 337–342.
Kiaris H, Spandidos DA, Jones AS, Vaughan ED, Field JK . (1995). Mutations, expression and genomic instability of the H-ras proto-oncogene in squamous cell carcinomas of the head and neck. Br J Cancer 72: 123–128.
Lewis WH, Yeger H, Bonetta L, Chan HS, Kang J, Junien C et al. (1989). Homozygous deletion of a DNA marker from chromosome 11p13 in sporadic Wilms’ tumor. Genomics 3: 25–31.
Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI et al. (1997). PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 275: 1943–1947.
Loughran O, Clark LJ, Bond J, Baker A, Berry IJ, Edington KG et al. (1997). Evidence for the inactivation of multiple replicative lifespan genes in immortal human squamous cell carcinoma keratinocyte. Oncogene 14: 1955–1964.
Mahoney PA, Weber U, Onofrechuk P, Biessmann H, Bryant PJ, Goodman CS . (1991). The fat tumor suppressor gene in Drosophila encodes a novel member of the cadherin gene superfamily. Cell 67: 853–868.
Pinkel D, Segraves R, Sudar D, Clark S, Poole I, Kowbel D et al. (1998). High resolution analysis of DNA copy number variation using comparative genomic hybridization to microarrays. Nat Genet 20: 207–211.
Pinkel D, Straume T, Gray JW . (1986). Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization. Proc Natl Acad Sci USA 83: 2934–2938.
Ponassi M, Jacques TS, Ciani L, Ffrench-Constant C . (1999). Expression of the rat homologue of the Drosophila fat tumour suppressor gene. Mech Dev 80: 207–212.
Prime SS, Nixon SVR, Crane IJ, Stone A, Matthews JB, Maitland NJ et al. (1990). The behaviour of human oral squamous cell carcinoma in cell culture. J Pathol 160: 259–269.
Rook MS, Delach SM, Deyneko G, Worlock A, Wolfe JL . (2004). Whole genome amplification of DNA from laser capture-microdissected tissue for high-throughput single nucleotide polymorphism and short tandem repeat genotyping. Am J Pathol 164: 23–33.
Sambrook J, Fritsch EF, Maniatis T . (1989). Molecular Cloning: A Laboratory Manual, 2 edn. Cold Spring Harbor Laboratory Press: New York, p 9.14.
Siegelmann-Danieli N, Ben-Izhack O, Hanlon A, Ridge JA, Stein ME, Khandelwal V et al. (2005). P53 alteration in oral tongue cancer is not significantly associated with age at diagnosis or tobacco exposure. Tumori 91: 346–350.
Simon R, Struckmann K, Schraml P, Wagner U, Forster T, Moch H et al. (2002). Amplification pattern of 12q13–q15 genes (MDM2, CDK4, GLI) in urinary bladder cancer. Oncogene 21: 2476–2483.
Tamura G, Yin J, Wang S, Fleisher AS, Zou T, Abraham JM et al. (2000). E-Cadherin gene promoter hypermethylation in primary human gastric carcinomas. J Natl Cancer Inst 92: 569–573.
Tanoue T, Takeichi M . (2005). New insights into Fat cadherins. J Cell Sci 118: 2347–2353.
Teni T, Pawar S, Sanghvi V, Saranath D . (2002). Expression of Bcl-2 and bax in chewing tobacco-induced oral cancers and oral lesions from India. Pathol Oncol Res 8: 109–114.
Veltman JA, Schoenmakers EF, Eussen BH, Janssen I, Merkx G, van Cleef B et al. (2002). High-throughput analysis of subtelomeric chromosome rearrangements by use of array-based comparative genomic hybridization. Am J Hum Genet 70: 1269–1276.
Watson KL, Justice RW, Bryant PJ . (1994). Drosophila in cancer research: the first fifty tumor suppressor genes. J Cell Sci Suppl 18: 19–33.
Wooster R, Bignell G, Lancaster J, Swift S, Seal S, Mangion J et al. (1995). Identification of the breast cancer susceptibility gene BRCA2. Nature 378: 789–792.
Acknowledgements
We thank the patients and their families for helping and participating in this work. We are grateful to Dr Norio Takahashi for performing preliminary experiments and helpful discussion about CGH array. This study was supported in part by grant-in-aids from the Ministry of Education, Culture, Sports, Science and Technology, Japan and from the Ministry of Health, Labor and Welfare of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakaya, K., Yamagata, H., Arita, N. et al. Identification of homozygous deletions of tumor suppressor gene FAT in oral cancer using CGH-array. Oncogene 26, 5300–5308 (2007). https://doi.org/10.1038/sj.onc.1210330
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210330
Keywords
This article is cited by
-
Detailed characterization of the transcriptome of single B cells in mantle cell lymphoma suggesting a potential use for SOX4
Scientific Reports (2021)
-
Overexpression of the giant FAT1 cadherin gene and its prognostic significance in de novo acute leukaemia patients
Comparative Clinical Pathology (2017)
-
Control of mitochondrial function and cell growth by the atypical cadherin Fat1
Nature (2016)
-
FAT1: a potential target for monoclonal antibody therapy in colon cancer
British Journal of Cancer (2016)
-
FAT4 functions as a tumour suppressor in gastric cancer by modulating Wnt/β-catenin signalling
British Journal of Cancer (2015)